Díaz Acedo R1, Mejías Trueba M2, Galván Banqueri M1, Saborido Cansino C1
1 Área de Gestión Sanitaria Sur de Sevilla (España)
2 Hospital Universitario Virgen del Rocío. Sevilla (España)
Fecha de recepción: 24/06/2020 – Fecha de aceptación: 14/08/2020
Correspondencia: Rocío Díaz Acedo – Hospital de Valme. Servicio de Farmacia (Área de Gestión Sanitaria Sur de Sevilla) – Ctra. de Cádiz, s/n – 41014 Sevilla (España)
diazacedo93@icloud.com
____
SUMMARY
Objectives: Andalusian Health Service (AHS) established the RP4 program, based on the review of potential prescription problems (PPPs) in order to improve patient’s safety. Some of these PPPs are related to kidney damage (PPPKDs). The main objective of this study is to evaluate the percentage of acceptance of the pharmaceutical intervention (PI) developed in a Health Management Area (HMA) for reducing PPPKDs. We also aimed to describe these PPPKDS and to analyze the evolution of these data between 2015-2019.
Methods: Retrospective study conducted by the Pharmacy Service of an HMA which offers health coverage to 406,768 patients. All the PPPKDs detected in these patients were included. Data were collected through an AHS Web Application. A descriptive analysis of variables was developed.
Results: In 2019, 466 PPPKDs (involving 460 patients) were detected. Overall percentage of acceptance of the PI was 90.7% and, according to type of PPPKD, was: 92.8% for NSAIDs duplication, 90.7% for double renin-angiotensin-aldosterone system (RAAS) blockade and 89.8% for triple Whammy.
During 2015-2019, detected PPPKDs have decreased from 634 to 466, and the percentage of acceptance has been adequate every year.
Conclusion: The acceptance of the PI, framed in the RP4 program, was optimal. The number of PPPKDs detected has decreased and the percentage of acceptance has remained elevated during the study period, which would support the utility of this program for the improvement of patients’ safety.
Key words: Drug prescription, drug safety, intervention study, NSAIDs, renin angiotensin aldosterone system.
Intervención multidisciplinar para reducir los posibles problemas de prescripción relacionados con el daño renal en pacientes crónicos
RESUMEN
Objetivos: El Sistema Andaluz de Salud (SAS) estableció el programa RP4, basado en la revisión de potenciales problemas de prescripción (PPPs) a fin de mejorar la seguridad de los pacientes. Algunos de los PPPs están relacionados con el daño renal (PPPKDs). El objetivo principal de este estudio es evaluar el porcentaje de aceptación de la intervención farmacéutica (PI) llevada a cabo en un Área de Gestión Sanitaria (HMA) para reducir los PPPKDs. Otros objetivos son describir estos PPPKDs y analizar la evolución de estos datos entre 2015-2019.
Métodos: Estudio retrospectivo desarrollado por el Servicio de Farmacia de un HMA que ofrece atención sanitaria a 406.768 pacientes. Todos los PPPKDs detectados en estos pacientes se incluyeron. Los datos se rcogieron a través de una aplicación web del SAS. Se realizó un análisis descriptivo de las variables.
Resultados: En 2019, se detectaron 466 PPPKDs (involucrando a 460 pacientes). El porcentaje global de aceptación de la PI fue del 90,7% y, según el tipo de PPPKD, fue: 92,8% para la duplicidad de AINEs, el 90,7% para el doble bloqueo del eje renina-angiotensina-aldosterona (RAAS) y del 89,7% para la triple Whammy.
Durante 2015-2019, los PPPKDs detectados han descendidio de 634 a 466 y el porcentaje de aceptación ha sido adecuado cada año.
Conclusión: La aceptación de la PI, enmarcada en el programa RP4, ha sido óptima. El número de PPPKDs detectado ha descendido y el porcentaje de aceptación se ha mantenido elevado durante el periodo de estudio, lo que podría avalar la utilidad de este programa para mejorar la seguridad de los pacientes.
Palabras clave: Prescripción de medicamentos, seguridad del medicamento, estudio de intervención, AINEs, eje renina-angiotensina-aldosterona.
____
INTRODUCTION
The Andalusian Health Service (AHS) established in 2012 a patient’s safety program (called RP4)1, which is based on the review of potential prescription problems (PPPs), in order to reduce the total number of prescriptions in polymedicated patients and some possible adverse effects related to those prescriptions, thus improving patient’s safety. In addition, this program can serve as a support tool for a more adequate prescription of certain drugs/pharmacological groups.
These PPPs are classified according to the different safety problems which can appear during these treatments, such as potential kidney damage. Potential prescription problems related to kidney damage (PPPKDs) are based in concomitant prescriptions of certain drugs which association is considered potentially nephrotoxic.
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in clinical practice2. However, several studies have found that the continued administration of these drugs, even more if combined, may contribute to the development of renal dysfunction2,3.
Renin-angiotensin-aldosterone system (RAAS) inhibitors are also considered as nephrotoxic drugs because of a higher incidence of acute renal failure (ARF) and hyperkalemia have been observed when two of them had been administered concomitantly4-6.
Diuretics also have nephrotoxic potential, especially associated with other drugs such as NSAIDs or RAAS inhibitors2. Many authors have defined the association of a RAAS inhibitor + a diuretic + a NSAID as “triple Whammy”, demonstrating the ability to trigger ARF in different clinical situations4,5,7.
Pharmacists’ notification of these PPPs to the physicians every year aims to improve the safety of the patients who are treated in the AHS. It could be possible if a good acceptance is obtained and detected PPPs are solved.
The main objective of this study, framed within the RP4 program, is to evaluate the percentage of acceptance of the pharmaceutical intervention (PI) about PPPKDs developed in a Health Management Area (HMA).
In addition, two secondary objectives are established:
- To describe the PPPKDs detected in this population.
- To evaluate the utility of the RP4 program, by analyzing the evolution (2015-2019) of the number of PPPKDs detected and the response of the physicians to the PI.
METHODS
Retrospective study conducted by the Pharmacy Service of a HMA, composed by a specialty hospital and 34 healthcare services, which offers health coverage to 406,768 patients. All the PPPKDs detected in these patients were included.
Three PPPKDs were defined:
- 1. NSAIDs duplication: concomitant prescription of two or more NSAIDs during a period longer than two months.
- 2. Double RAAS blockade: concomitant prescription of an angiotensin-converting enzyme (ACE) inhibitor plus an angiotensin-II receptor blocker (ARB) or an ACE inhibitor/ARB plus another different RAAS inhibitor.
- 3. Triple Whammy: concomitant prescription of one NSAID, one diuretic and one RAAS inhibitor.
Annually, the Pharmacy Service participates in the RP4 program according to the following procedure:
- 1. The AHS send to the Pharmacy Service a list of the patients affected by PPPs.
- 2. This patients’ list is made available to doctors through an AHS Web Application, through which a response to the PI is requested to these professionals.
- 3. Later, prescribers start to review notified prescriptions.
This study analyzes the physicians’ response to the PI and the PPPKDs detected during 2019. In addition, it also analyzes the evolution of the detected PPPKDs and the physicians’ response to PI, between 2015 and 2019.
Demographic variables [age, polymedication (yes/no)] from patients affected by PPPKDs in 2019 were collected. Patients were considered as polymedicated when they had more than five concomitant prescriptions.
The percentage of acceptance of the PI was established as main variable.
For analyzing this variable, three categories were defined:
- 1. Modified prescription: when the physician suspended or adapted the treatment, achieving the resolution of the PPP.
- 2. Justified prescription: when the physician decided to maintain the initial prescription because it was considered appropriate for the patient’s clinical situation.
- 3. Non-reviewed prescription: when physicians did not send any feedback.
Accepted PI was considered when notified PPPKDs had been reviewed by the physicians (justified or modified prescriptions).
Four secondary variables were established: Physicians’ reasons for justifying/modifying prescriptions, number and type of PPPKDs detected between 2015-2019 and physician’s response to the PI during 2015-2019.
Data were collected through an AHS Web Application. A descriptive analysis of variables was developed. For quantitative variables, mean and standard deviation were calculated. For qualitative variables, measures of absolute, relative frequency and percentages were used. Analyses were performed using the statistical package IBM SPSS Statistic®.
RESULTS
The PI developed during 2019 addressed 460 patients involved in 466 PPPKDs, with a mean age of 65.8±14.3 years and a percentage of polymedication of 55.7%. Basal characteristics of the study population are shown in table 1.
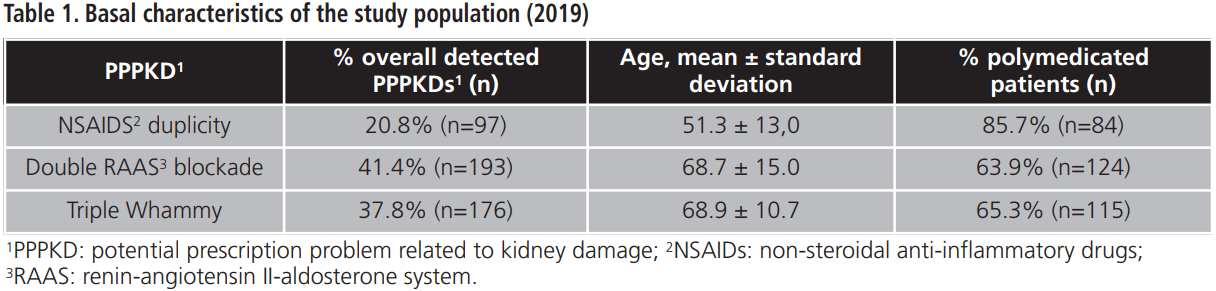
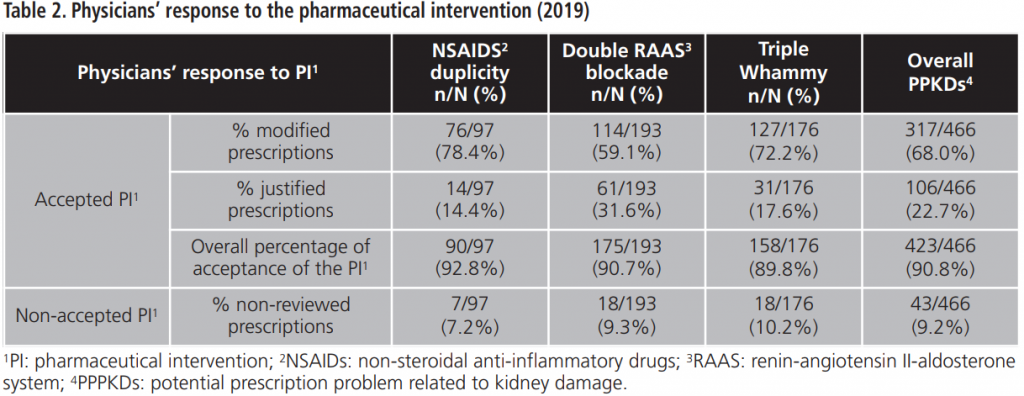
The overall percentage of acceptance of the PI was 90.7%. The percentage of acceptance according to type of PPPKD was 92.8% for NSAIDs duplication, 90.7% for double RAAS blockade and 89.8% for triple Whammy. Tables 2-4 details doctors’ response to PPPKDs.
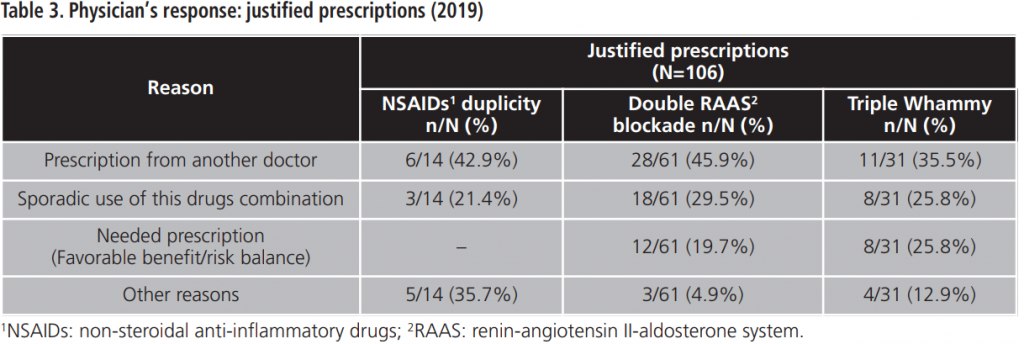
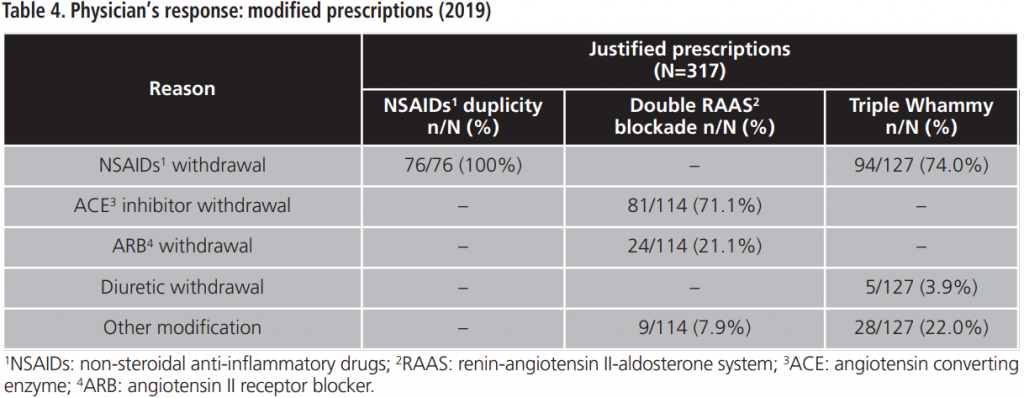
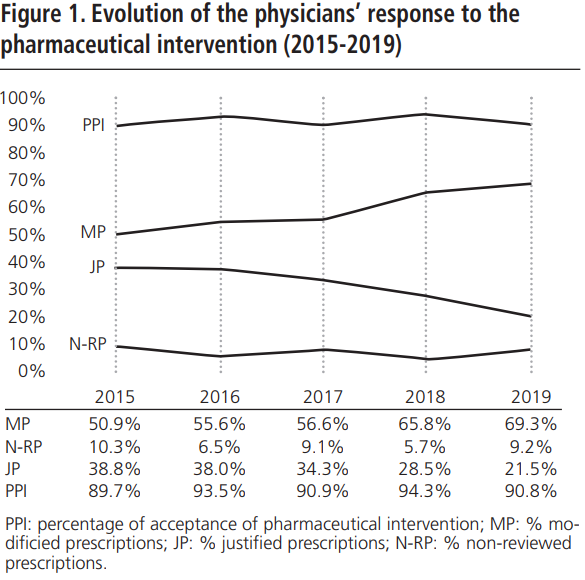
During 2015-2019, an annual mean of 530.6±64.8 PPPKDs was detected, present in a mean of 526.4±64.3 patients. The most frequently detected problem was the double RAAS blockade. Table 5 collects PPPKDs detected every year.
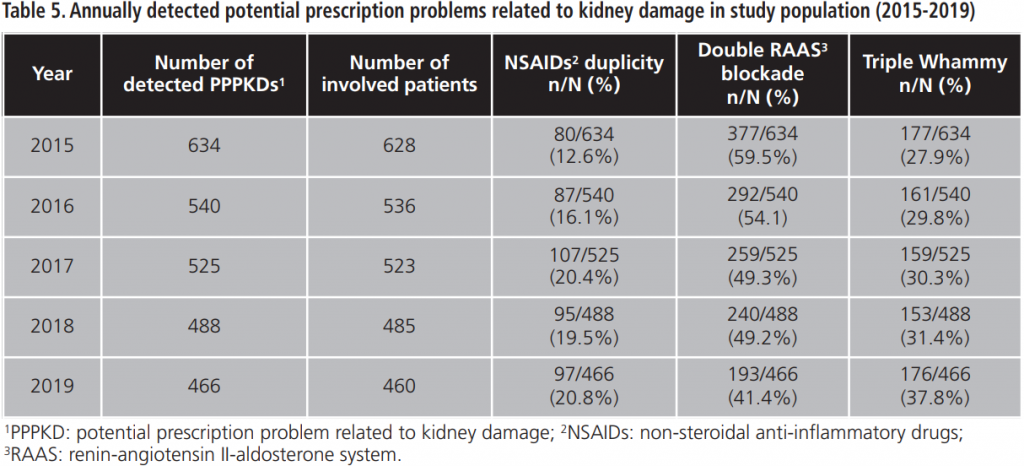
The percentage of acceptance of the PI between 2015-2019 was 91.8%±1.9. The evolution of the physicians’ response to the PI during the study period is shown in figure 1.
All these drugs combinations have widely demonstrated their ability to precipitate ARF or to worse chronic renal failure2. In addition, the mean age of the affected patients is over to 65 years and more than a half are polymedicated, so this fragile population could have previous renal dysfunction and/or additional prescription of other nephrotoxic drugs not included in this study.
In this study, the overall acceptance of the PI is optimal and higher than acceptance rates observed in other papers8,9. The lowest percentage of acceptance is observed for the triple Whammy, probably because doctors priorize to review another PPPKDs based on drugs duplications, which are more frequently related to prescription mistakes. However, a high percentage of the reviewed prescriptions for triple Whammy was modified and, in most cases, the NSAID was withdrawn. This could be explained because the treatment with one diuretic plus one RAAS inhibitor is indicated in several cardiovascular pathologies according to main clinical practices guideline10,11, although this association has been related by other authors with a high percentage of development of out-of-hospital ARF7.
The most detected PPPKD among our study population was double RAAS blockade. Many physicians justified the prescription of two RAAS inhibitors because one of them was captopril, an ACE inhibitor with rapid action and short half-life, which is prescribed for punctual use in hypertensive crisis12,13.
Twelve prescriptions of double RAAS blockade were justified because the patient had an uncontrolled clinic situation. Although this association is not widely recommended for safety reasons, some authors have observed better efficacy data when an ACE inhibitor and an ARB are associated in patients with heart failure, although an increased renal toxicity has been documented6.
Regarding the percentage of acceptance among NSAIDs duplication, most of prescriptions were modified, so it could be translated into an improvement of patients’ safety.
Throughout the study period (2015-2019), the number of PPPKDs detected has decreased and the acceptance of the PI has remained elevated, which highlights the utility of the RP4 program to serve as a support tool for the prescription of drugs from these pharmacological groups.
As limitations of the study, to highlight that, since the review phase is developed a few months after the pharmacist`s notification, some of the PPPKDs detected may have been previously solved or new PPPKDs may have appeared and they will not be detected until next year. In addition, in order to assess the real utility of the RP4 program with greater evidence, it might be convenient to analyze other parameters in the study population to verify that these modifications in prescription really have a positive impact on patients’ health.
CONCLUSION
Taking into account the above limitations, we can conclude that the acceptance of the PI, framed in the RP4 program, is adequate. The number of detected PPPKDs has decreased and the percentage of acceptance has remained elevated over the years, which would support the utility of this program for the improvement of patients’ safety. However, in order to achieve a greater decrease in the number of detected PPPKDs, other complementary activities focused on prescribers could be implemented.
Conflict of interests: The authors declare that they do not present a conflict of interest.
____
BIBLIOGRAPHY
1. Centro Andaluz de Documentación e Información de Medicamentos (CADIME). (2017) Revisión de prescripciones para evitar problemas de seguridad. Boletín terapéutico andaluz, 32(1). Available in: https://dx.doi.org/10.11119/ BTA2017-32-01.
2. Szeto C, Sugano K, Wang J, Fujimoto K, Whittle S, Modi G, et al. (2020) Non-steroidal anti-inflammatory drug (NSAID) therapy in patients with hypertension, cardiovascular, renal or gastrointestinal comorbidities: joint APAGE/ APLAR/ APSDE/APSH/APSN/PoA recommendations (Internet). Gut, 0:1-13. (consulted 24-01-2020). Availaible in: https://www.ncbi.nlm.nih.gov/pubmed/31937550.
3. Zhang X, Donnan P, Bell S, Guthrie B. (2017) Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic disease: systematic review and meta-analysis. BMC Nephrology. 18(1):256.
4. Rivosecchi R, Kellum J, Dasta J, Armahizer M, Bolesta S, Buckley M, et al. (2016) Drug Class combination- associated acute kidney injury. Annals of Pharmacotherapy. 50(11):953-72.
5. Fried L, Emanuel N, Zhang J, Brophy M, Conner T, Duckworth W, et al. (2013) Combined Angiotensin Inhibition for the treatment of diabetic nephropathy. New England Journal of Medicine. 369(20):1892-903.
6. McMurray J, Ostergren J, Swedberg K, Granger C, Held P, Michelson E, et al. (2003) Effects of candersartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. The Lancet. 362:767-71.
7. García-Camin R, Cols M, Chevarria J, García-Osuna R, Carreras M, Lisbona J, et al. (2015) Fracaso renal agudo secundario a combinación de inhibidores del sistema renina-angiotensina, diuréticos y AINES (La triple Whammy). Nefrologia. 35(2):197-2016.
8. Ho MJ and Venci J. (2012) Improving the success of mailed letter intervention programs to influence prescribing behaviors: a review. Journal of Managed Care & Specialty Pharmacy. 18(8):627-49.
9. Perera P, Guy M, Sweaney A and Boesen K. (2011) Evaluation of prescriber responses to pharmacist recommendations communicated by fax in a medication therapy management program (MTMP). Journal of Managed Care & Specialty Pharmacy. 17(5):345-54.
10. Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. (2019) Guía ESC/ESH 2018 sobre el diagnóstico y tratamiento de la hipertensión arterial. Revista Española de Cardiología. 72(2):160-78.
11. Seferovic P, Ponikowsky P, Anker S, Bauersachs J, Chioncel O, Cleland J, et al. (2019) Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patients management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. European Journal of Heart Failure. 21(10):1169-86.
12. Kazerani H, Hajimoradi B, Amini A, Naseri M, and Moharamzad Y. (2009) Clinical efficacy of sublingual captopril in the treatment of hypertensive urgency. Singapore Medicine Journal. 50(4):400.
13. Kaya A, Tatlisi M, Kaya T, Yildirimturk O, Gungor B, Karatas B, et al. (2016) Sublingual vs Oral Captopril in Hypertensive crisis. Journal of Emergency Medicine. 50(1):108-15.
____
