Fecha de recepción: 11/10/2023. Fecha de aceptación: 14/11/2023
Sopena Carrera L 1,Magallón Martínez A 1, Garcés Redondo M2, Fresquet Molina R1, Merchán Flores A1, Salvador Gómez T1
1. Department of Pharmacy, Lozano Blesa University Clinical Hospital, Zaragoza (Spain)
2. Department of Neurology, Lozano Blesa University Clinical Hospital, Zaragoza (Spain)
Correspondencia: luciasopenacarrera@gmail.com
____
Abstract
Background: Patient adherence in multiple sclerosis is crucial for treatment success.
Aim: Primary: To analyze the level of adherence among multiple sclerosis patients who collected disease-modifying therapies from the Pharmacy Department of a Spanish hospital. Secondary: To determine if patient adherence to treatment is influenced by demographic, clinical, and treatment-related characteristics.
Method: Retrospective and single-center observational study. Study population: multiple sclerosis patients with active disease-modifying therapies in 2021 who collected their treatment from the Pharmacy Department. Patient adherence was determined over a 12-month period using the Medication Possession Ratio. Bivariate and multivariate analyses were performed to study if any variables influenced patient adherence to their treatment.
Results: A total of 214 patients (62.1% females) were included. The median age at the start of treatment was 44 years, and 32 years at the time of diagnosis. Ninety-five point three percent of the patients had relapsing-remitting multiple sclerosis, and the median disease duration was 11 years. The most commonly used route of administration was oral, and the most prescribed drug was teriflunomide. Ninety point nine percent of the patients had good adherence, and it was found that the oral route was associated with good adherence, while subcutaneous interferon beta-1b was associated with poor adherence.
Conclusion: Our population has similar clinical baseline characteristics to the general multiple sclerosis population. In our study, the majority of multiple sclerosis patients are adherent to their treatment, and the route of administration and type of medication influence adherence.
Keywords: Multiple Sclerosis, Therapeutics, Medication Adherence, Drug administration Routes, Interferon beta-1b, Quality of life.
Análisis de la adherencia y de los factores de riesgo asociados en los pacientes con esclerosis múltiple bajo terapia modificadora de la enfermedad
Resumen
Antecedentes y justificación: La adherencia de los pacientes con esclerosis múltiple (EM) es clave para el éxito en el tratamiento.
Objetivo: Primario: Analizar el grado de adherencia de los pacientes con EM que acudieron a recoger los tratamientos modificadores de la enfermedad (TME) al Servicio de Farmacia de un hospital español. Secundario: determinar si la adherencia de los pacientes al tratamiento está influenciada por características demográficas, clínicas y del tratamiento.
Material y métodos: Estudio observacional retrospectivo y unicéntrico. Población a estudio: pacientes con EM con TME activo durante el año 2021 que acudieron a recoger su tratamiento al Servicio de Farmacia. Se determinó la adherencia de los pacientes en un periodo de 12 meses mediante la Medication Possession Ratio (MPR). Se estudió mediante un análisis bivariante y multivariante si alguna de las variables influía en la adherencia de los pacientes a su tratamiento.
Resultados: Se incluyeron 214 pacientes (62,1% mujeres). La mediana de edad fue de 44 años al inicio de tratamiento y de 32 años en el momento del diagnóstico. El 95,3% de los pacientes presentaban EM recurrente-remitente y la mediana de tiempo de evolución de la enfermedad fue de 11 años. La vía de administración más usada fue la oral y el fármaco más prescrito la teriflunomida. El 90,9% de los pacientes tuvo una buena adherencia y se halló que la vía oral estaba asociada con una buena adherencia y el interferón beta 1b subcutáneo con una mala adherencia.
Conclusiones: Nuestra población presenta unas características clínicas basales similares a las de la población general con EM. En nuestro estudio, la mayoría de los pacientes con EM son adherentes a su tratamiento, y la vía de administración y el tipo de fármaco influyen en la adherencia.
Palabras clave: Esclerosis Múltiple, Terapéutica, Adherencia de la medicación, Vías de administración de fármacos, Interferon beta-1b, Calidad de vida.
_____
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating, and degenerative disease of the central nervous system (CNS), caused by the loss of myelin from oligodendrocytes1,2. It is one of the leading causes of disability in young adults, affecting 3.5 women for every man3. It is estimated that 2.5 million people worldwide suffer from MS. MS has different disease courses depending on whether patients experience relapses alternating with periods of remission or a progressive deterioration4: clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), and primary progressive MS (PPMS).
To assess the degree of disability throughout the course of the disease, the Expanded Disability Status Scale (EDSS) is used, which evaluates the functional status of patients and their degree of disability in performing daily activities based on the involvement of eight functional systems5,6.
While there is no cure for MS, there are effective strategies to treat exacerbations (relapses), modify the course of the disease, and manage symptoms. Disease-modifying therapies (DMTs) can help patients control their MS and improve their comfort and quality of life.
Good therapeutic adherence is key to treatment success. Adherence rates to DMTs in MS range from 49% to 88%7. It has been shown that patients with MS who have good adherence have a lower risk of relapse, fewer emergency visits, fewer severe relapses, fewer hospitalizations, fewer neuropsychological problems, lower costs, and higher quality of life. However, poor adherence has been associated with increased mortality and higher healthcare costs8. Various studies have demonstrated that multiple factors can affect the adherence of patients with MS undergoing DMTs: patient-related factors, disease status factors, and medication-related factors7-9.
There are different methods to assess adherence. The most commonly used measure for adherence based on dispensing records is the Medication Possession Ratio (MPR), which is defined as the number of days the patient possesses the medication divided by the number of days the patient should possess the medication. Most current studies on adherence define good adherence as an MPR value ≥ 0.810-12.
Aim
The main objective of this study is to analyze the level of adherence among patients with MS who collected DMTs from the Pharmacy Department of a tertiary Spanish hospital in 2021. As a secondary objective, we aimed to determine if patient adherence to treatment was influenced by demographic and clinical characteristics, disease status, and medication (drug, route of administration, and dosage).
Ethics approval
All information was treated as confidential and used exclusively in a professional setting. The study participants were anonymized using a code in all reports and data analyses. This study received approval from the Aragon Clinical Research Ethics Committee (CEICA) on 3 May 2022 (EPA22/022).
Materials and Methods
This was a retrospective, single-center observational study of patients with active MS receiving DMTs in the year 2021, who collected their treatment from the hospital’s Pharmacy Department. Patients receiving subcutaneous (sc) interferon beta-1b, intramuscular (im) interferon beta-1a, sc interferon beta-1a, teriflunomide, dimethyl fumarate, and fingolimod were included. Inclusion criteria were a diagnosis of MS according to the McDonald criteria13, age above 18 years, and active treatment with one of the drugs proposed in the study. Exclusion criteria were lack of follow-up or data. Demographic variables collected included sex, age at the start of treatment, and age at diagnosis. Clinical variables included MS type, disease duration, degree of disability during the study period based on EDSS scores, relapses (yes/no), number of relapses in previous years, hospitalizations (yes/no), number of hospitalizations in previous years, and comorbidities and disease symptoms. Pharmacological variables collected included route of administration, type of drug, treatment line, treatment duration, polypharmacy (defined as > 5 drugs per day), adverse effects (AEs) to DMTs, and adherence.
Data were extracted from the Outpatient Dispensing Program used in the Pharmacy Department and the Electronic Health Record (HER). All collected variables were obtained directly from the mentioned sources of information. However, adherence was indirectly obtained through MPR, considering the dosage of each drug, with good adherence defined as an MPR value ≥ 0.8.
For statistical analysis, a descriptive study of the sample was conducted using absolute and relative frequencies (percentages) for qualitative variables, and measures of central tendency and dispersion for quantitative variables. Normality tests, including the Kolmogorov-Smirnov test (n>30), were performed to determine if the variables followed a normal distribution. Normality was also assessed using Q-Q plots and the test of equality of variances (Levene’s test). Mean (standard deviation) was used to express central tendency for normally distributed variables, while median (range) was used for non-normally distributed variables. The level of statistical significance was set at 5% two-tailed. Next, an analytical study was conducted to evaluate if there was a statistically significant association between the included variables (independent variables) and adherence (dependent variable). Bivariate analysis was performed, considering a p-value < 0.05 as statistically significant. For qualitative variables, the chi-square test was used, and in cases where conditions were not met, Fisher’s exact test was used. For normally distributed quantitative variables, the parametric independent samples t-test was used, while the non-parametric Mann-Whitney U test was used for variables that did not follow a normal distribution. The strength of association was measured using Phi and Cramer’s V coefficients. The direction of the association was measured through Yule’s Q coefficient for dichotomous variables and Haberman’s adjusted standardized residuals for polytomous variables.
Finally, a multivariate logistic regression analysis was performed, with adherence as the outcome variable and demographic, clinical, and pharmacological variables that were statistically significant in the bivariate analyses, as well as those considered clinically relevant, as explanatory variables. The goodness of fit of the multivariate models was evaluated using the Nagelkerke and Hosmer-Lemeshow tests.
Results
A total of 214 patients were included, with 62.1% being women (n=133). The median age at the start of treatment was 44 (18 – 66) years, and the median age at diagnosis was 32 (7 – 55) years. Ninety-five point three percent of the patients had RRMS (n=204), while 4.7% had SPMS (n=10). None had PPMS. The median disease duration was 11 (0.2 – 45) years, and the median EDSS score was 1.5 (0 – 8). Fifty-one point four percent (n=110) of the patients had experienced at least one relapse in the years prior to starting DMTs, with a median number of relapses of 2 (0 – 17). Thirty-nine point three percent (n=84) of the patients had at least one hospitalization in the years prior to treatment, with a median number of hospitalizations of 0 (0 – 4).
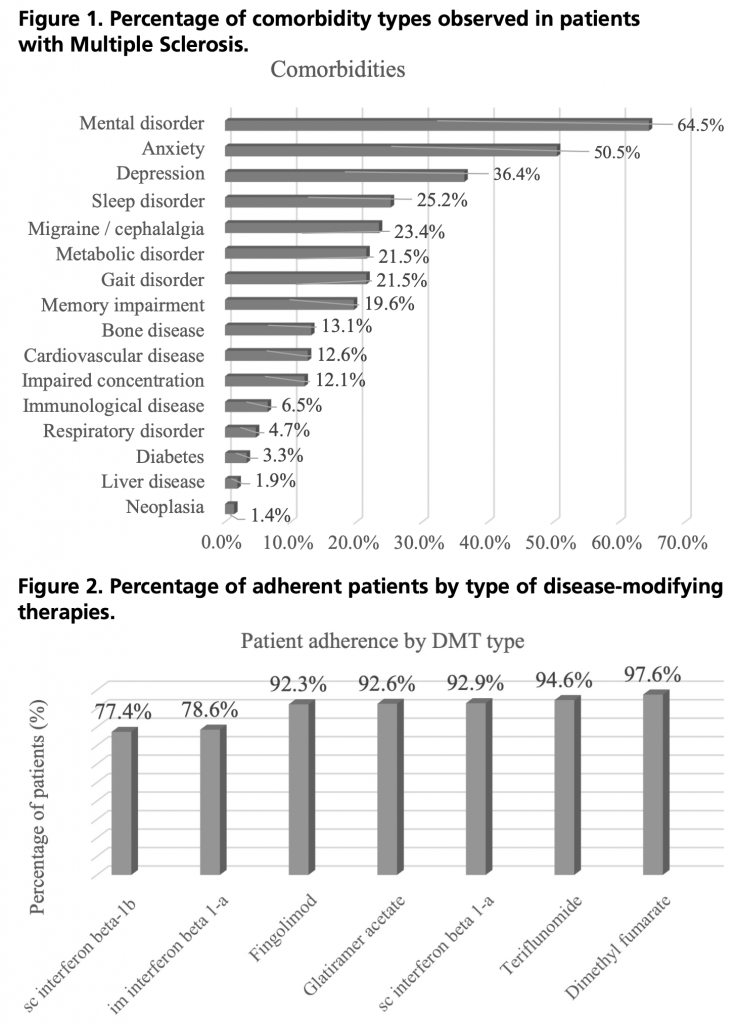
Seventy point nine percent of the patients had comorbidities and symptoms related to the disease. Figure 1 displays the rates of comorbidities by type.
Regarding treatment, oral DMTs were prescribed in 59.3% (n=127) of the patients, while injectable DMTs were prescribed in 40.7% (n=87). Among the total of 214 patients, the most prescribed drug was teriflunomide (26.6%, n=57), followed by dimethyl fumarate (20.6%, n=44), sc interferon beta-1b (14.5%, n=31), glatiramer acetate (12.6%, n=27), fingolimod (12.1%, n=26), im interferon beta-1a (7.0%, n=15), and finally sc interferon beta-1a (6.5%, n=14). The median treatment line was 2 (1 – 5), and the median treatment duration during the follow-up period was 63.8 (1.2 – 300.9) months. Thirty-eight point three percent (n=82) of the patients were polymedicated.
In terms of safety, 53.7% (n=115) of the patients experienced some type of AE related to the studied DMTs.
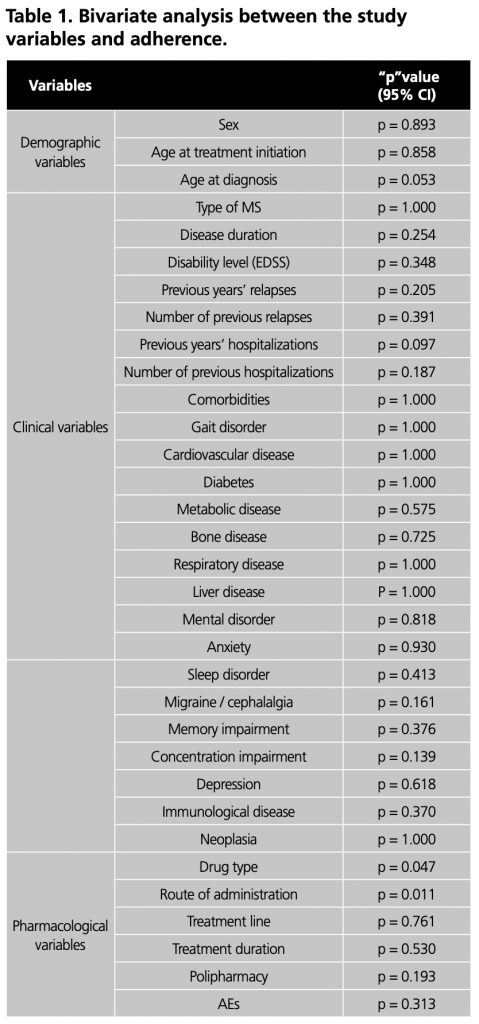
Regarding adherence, 90.9% of the patients achieved a MPR ≥ 0.8, while 9.1% had a MPR ≤ 0.8. The median MPR was 1 (0.2 – 1). Among patients using injectable DMTs, 84.9% were adherent, while among patients using oral DMTs, the adherence percentage was 95.1%. Figure 2 provides a breakdown of the percentage of adherent patients (MPR ≥ 0.8) by type of DMT.
The analysis of the relationship between demographic, clinical, and pharmacological variables and treatment adherence is shown in Table 1.
A statistically significant association was found between the “route of administration” variable and adherence (95% CI p = 0.011). This association was weak (Phi and Cramer’s V < 0.3) but significant (p < 0.05). It was demonstrated that the oral route was associated with good adherence (Yule’s Q = 0.55), while the injectable route was associated with poor adherence (Yule’s Q = -0.55). A statistically significant association was also found between the “type of drug” variable and adherence (95% CI p = 0.047). This association was weak (Phi and Cramer’s V < 0.3) but significant (p < 0.05).
Furthermore, a statistically significant association was found between patients treated with “sc interferon beta-1b” and poor adherence (Haberman’s residuals > 1.96). No statistically significant association was found for the other drugs (Haberman’s residuals between -1.96 and 1.96). No statistically significant associations were found for the remaining variables (Table 1).
For the multivariate analysis, the explanatory variables included the route of administration and the type of drug, as they were statistically significant in the bivariate model, and adjusted for sex and age, considered to be clinically relevant (Table 2).
A statistically significant association was found for im interferon beta-1a (p = 0.043) and sc interferon beta-1b (p = 0.022), both administered by the parenteral route (Table 2).
Discussion
The main objective of this study was to analyze the level of adherence over a 12-month period in patients with MS who came to collect their DMTs at the Pharmacy Department. Secondly, we determined whether the adherence of these patients to DMTs was influenced by demographic and clinical characteristics, disease status, and the specific drug. Intravenous DMTs like natalizumab and ocrelizumab were not included in the study as they are administered in the hospital. Cladribine was also excluded due to its dosing schedule of twice a year. Siponimod was not included either because it was recently commercialized at the time of data collection for this study.
Regarding the demographic characteristics of our population, a female predominance in MS can be objected (62.1% women). This finding is consistent with existing evidence8,12,14-17, although the literature mentioned reports a percentage of over 70% women.
The age at treatment initiation in our study was 44 (18 – 66) years, and the age at diagnosis was 32 (7 – 55) years. This aligns with the age described in the available literature8,16,17.
A high percentage of patients had comorbidities (70.9%). We found that 64.5% of MS patients also had some form of mental disorder, with anxiety being the most prevalent (50.5%), followed by depression (36.4%). A study conducted in the US by Michael Munsell et al.8 analyzed the comorbidities in patients with MS, and the most prevalent ones were consistent with our study, with anxiety being the primary comorbidity. In 2021, Gisela Zanaga et al.7 reported that 43% of patients had some form of comorbidity in addition to MS, with depression being present in 33% of cases.
Polypharmacy is a reality for MS patients on DMTs. In this study, 38.3% of patients were receiving multiple medications. Our result is consistent with the study by Gisela Zanaga et al.7. However, a study published in 2019 by Niklas Frahm et al.18, which aimed to evaluate the frequency of polypharmacy among MS patients, reported a higher percentage (56.6%).
In this study, we found that more than half of the patients (53.7%) experienced some form of AEs. Our result is considerably higher than the one reported by Gisela Zanaga et al.7, who found a 28.0% rate of AEs.
The level of adherence to treatment was measured using MPR. The majority of patients included in the study were considered adherent to treatment (90.9%). Several studies have assessed adherence in MS patients using MPR and considered an MPR ≥ 0.8 as good adherence. However, those studies reported lower rates of good adherence, ranging from 70% to 80%16,17,19,20. Our result is much higher in comparison.
In this study, oral administration showed a higher percentage of adherence compared to injectable administration. Regarding drug-specific adherence, dimethyl fumarate had the highest adherence rate, followed by teriflunomide, sc interferon beta-1a, glatiramer acetate, fingolimod, im interferon beta-1a, and sc interferon beta-1b.
After bivariate and multivariate analysis, we found that the route of administration influenced patient adherence, with oral administration associated with better adherence and parenteral administration associated with poorer adherence. We also found a statistically significant association with the type of drug, specifically, patients receiving sc interferon beta-1b showed poorer adherence. However, we could not find statistically significant associations for the rest of the drugs.
Regarding demographic variables, this study did not find a significant association between these variables and adherence. Other authors also did not find statistically significant associations between demographic variables and adherence7,16,17. However, other studies have
demonstrated that younger age groups exhibit poorer adherence7,8,9,19,20.
Regarding clinical variables, this study did not find a statistically significant association between any of them and adherence. These results align with those of Gisela Zanaga et al.7, who also did not find significant differences in adherence based on disease duration, previous relapses or hospitalizations, comorbidities, or disability scale. Efrat Neter et al.16also did not find significant differences between disease duration, disability, and adherence.
In this study, we could not find a statistically significant association with the presence of comorbidities or disease symptoms. Our result coincides with that of Gisela Zanaga et al.7. However, other studies have shown a significant influence of comorbidities on adherence8,9,16,21.
Regarding pharmacological variables, there are studies that support our results. A recent meta-analysis conducted by Joshua Mardan et al. in 202122, which reviewed 46 articles analyzing variables that could influence adherence, found a significant association with the route of administration, with oral administration showing better adherence (91.0%) compared to injectable administration (77.0%) over a 12-month period. Other studies have also found higher adherence with oral drugs compared to injectables7,21,23,24.
Furthermore, in this study, we found that the type of drug influenced adherence, as reported in other studies in the literature12,25,26. In our study, sc interferon beta-1b was associated with poorer adherence. Our result is consistent with the findings of Emma Bartolomé-García et al.27 and Lukasz Jernas et al.28.
This study did not find a statistically significant association with treatment duration. Our result aligns with the study by Efrat Neter et al.16. We also could not find a significant association with polypharmacy and AEs, similar to the study by Gisela Zanaga et al.7, which also included these two variables.
Strengths and weaknesses
Regarding the limitations of this study, adherence was measured using the MPR method based on dispensing records analysis. This method assumes that medication replenishment by the patient corresponds to actual medication intake and that the medication is taken as prescribed. However, we cannot assert that patient adherence to medication implies correct intake or administration. Therefore, adherence may be overestimated. Nevertheless, numerous studies analyzing adherence in MS patients have used the MPR method, considering an MPR ≥ 0.8 as good adherence7,8,15,16,20.
Furthermore, this study did not include patient variables that could affect adherence due to lack of information in the EHR, such as socio-economic status, demographic region, education level, marital status, and family environment, which are included in other studies7,8,14,16.
However, this study has the following strengths: the inclusion of variables that are rarely found in published adherence predictor studies, such as MS type, treatment line, polypharmacy, and AEs. Additionally, this study analyzed the adherence of seven DMTs, whereas previous studies analyzed fewer drugs.
Further research
The study’s findings provide healthcare providers with valuable guidance when considering treatment options for MS patients. Identifying the most commonly prescribed drug and its association with adherence allows for personalized medicine approaches. This understanding can inform the development of individualized treatment plans, enhancing adherence rates and, consequently, improving patient outcomes.
However, further research is needed to explore additional factors that may influence adherence in this patient population. By continuing to investigate adherence behaviors among MS patients, we can strive to develop more effective strategies that improve treatment compliance and ultimately benefit the overall well-being of individuals living with this chronic condition.
Conclusion
This study aimed to analyze the adherence level of MS
patients over a 12-month period, specifically focusing on patients collecting their DMT from the Pharmacy Department.
Our population has similar baseline clinical characteristics to the general population with MS.
We found a high adherence rate among the included patients, with oral administration showing better adherence compared to injectables. The type of drug was also associated with adherence, with sc interferon beta-1b showing poorer adherence. Demographic and clinical variables did not show statistically significant associations with adherence in this study.
These findings contribute to the understanding of adherence patterns in MS patients, providing insights for healthcare professionals to optimize treatment outcomes. Further research is warranted to explore additional factors that may influence adherence in this patient population.
Acknowledgements: The author wishes to express their sincere gratitude to Arantxa Magallón for her unwavering dedication and valuable time devoted to this work, to Moisés Garcés for generously sharing his extensive knowledge and expertise, and to the head of the Department, Tránsito Salvador, for her support and availability whenever needed.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Haider L, Simeonidou C, Steinberger G, et al. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J Neurol Neurosurg Psychiatry. 2014 Dec;85(12):1386-1395. Available from: https://pubmed.ncbi.nlm.nih.gov/24899728/
- Calabrese M, Magliozzi R, Ciccarelli O, et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015 Feb;16(3):147-158. Available from: https://pubmed.ncbi.nlm.nih.gov/25697158/
- Pérez-Carmona N, Fernández-Jover E, Sempere Á. Epidemiology of multiple sclerosis in Spain. Rev Neurol. 2019;69(1):32-38.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83:278-286. Available from: https://pubmed.ncbi.nlm.nih.gov/24871874/
- Rodríguez A, Moral E, Mendibe M, et al. Guía oficial de práctica clínica en esclerosis múltiple. Luzán 5; 2014. Available from: https://www.sen.es/pdf/guias/Guia_oficial_de_practica_clinica_en_esclerosis_multiple_2014.pdf
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983;33:1444-1452.
- Zanga G, Drzewiscki E, Tagliani P, et al. Predictors of adherence and persistence to disease-modifying therapies in multiple sclerosis. Ther Adv Neurol Disord. 2021;14.
- Munsell M, Frean M, Menzin J, et al. An evaluation of adherence in patients with multiple sclerosis newly initiating treatment with a self-injectable or an oral disease-modifying drug. Patient Prefer Adherence. 2017;11:55-62.
- Gromisch ES, Turner AP, Leipertz SL, et al. Risk Factors for Suboptimal Medication Adherence in Persons With Multiple Sclerosis: Development of an Electronic Health Record-Based Explanatory Model for Disease-Modifying Therapy Use. Arch Phys Med Rehabil. 2020;101(5):807-814.
- Pagès-Puigdemont N, Valverde-Merino MI. Métodos para medir la adherencia terapéutica. Ars Pharmaceutica. 2018;59(3). Available from: https://doi.org/10.30827/ars.v59i3.7387
- López-Romero LA, Romero-Guevara SL, Parra DI, et al. Adherencia al tratamiento: concepto y medición. Hacia La Promoción de La Salud. 2016;117-137. Available from: https://doi.org/10.17151/hpsal.2016.21.1.10
- Kozma C, Dickson M, Phillips AL. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence. 2013;7:509. Available from: https://doi.org/10.2147/ppa.s40736
- Ward M, Goldman MD. Epidemiology and Pathophysiology of Multiple Sclerosis. 2022.
- Li P, Ladage VP, Berger J, et al. Disease-Modifying Therapy Adherence and Associated Factors in a National Sample of Medicare Patients With Multiple Sclerosis. Value Health. 2020;23(3):328-334.
- Groeneweg M, Forrester SH, Arnold B, et al. One-Year Outcomes of an Integrated Multiple Sclerosis Disease Management Program. J Manag Care Spec Pharm. 2018;24. Available from: www.jmcp.org
- Neter E, Glass-Marmor L, Wolkowitz A, et al. Beliefs about medication as predictors of medication adherence in a prospective cohort study among persons with multiple sclerosis. BMC Neurol. 2021;21:21.
- Evans C, Marrie RA, Zhu F, et al. Adherence and persistence to drug therapies for multiple sclerosis: A population-based study. Mult Scler Relat Disord. 2016;8:78-85.
- Frahm N, Hecker M, Zettl UK. Multi-drug use among patients with multiple sclerosis: A cross-sectional study of associations to clinicodemographic factors. Sci Rep. 2019;9:14677.
- Burkhard A, Toliver J, Rascati K. Association between multiple sclerosis disease severity and adherence to disease-modifying therapies. J Manag Care Spec Pharm. 2021;27:821-830.
- Melesse DY, Marrie RA, Blanchard JF, et al. Persistence to disease-modifying therapies for multiple sclerosis in a Canadian cohort. Patient Prefer Adherence. 2017;11:1093-1101.
- Higuera L, Carlin CS, Anderson S. Adherence to Disease-Modifying Therapies for Multiple Sclerosis. J Manag Care Spec Pharm. 2016;22(12):1399-1410.
- Mardan J, Mohammad B, Hussain A, et al. Objective medication adherence and persistence in people with multiple sclerosis: a systematic review, meta-analysis, and meta-regression. Mult Scler Relat Disord. 2021;55:103238.
- Lahdenperä S, Soilu-Hänninen M, Kuusisto HM, et al. Medication adherence/persistence among patients with active multiple sclerosis in Finland. Acta Neurol Scand. 2020;142(6):605-612.
- Sánchez Martínez I, Cerdán M, López J, et al. Possible Influence of the Route of Treatment Administration on Treatment Adherence in Patients With Multiple Sclerosis. Clin Ther. 2020;42(5):e87-e99.
- López-Méndez P, Río J, Pérez-Ricart A, et al. Adhesión terapéutica a tratamiento inmunomodulador de pacientes con esclerosis múltiple. Rev Neurol. 2013;56(01):8-12.
- Agashivala N, Wu N, Abouzaid S, et al. Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol. 2013;13:138.
- Bartolomé-García E, Usarralde-Pérez Á, Sanmartín-Fenollera P, et al. Persistence and adherence to interferon and glatiramer acetate in patients with multiple sclerosis. Eur J Hosp Pharm. 2019;26(1):23-28.
- Jernas L, Wencel J, Wiak A, et al. Risk factors for poor Adherence to Betaferon® treatment in patients with relapsing-remitting multiple sclerosis or clinically isolated syndrome. PLoS One. 2016;11(10):e0165193.
____