Rev. OFIL 2017, 27;4:398-400
Fecha de recepción: 20/01/2017 – Fecha de aceptación: 26/01/2017
Morales-Molina JA, González-Vaquero D, Martínez-de la Plata JE, Fernández-Martín JM
Hospital de Poniente. Agencia Pública Sanitaria Poniente. El Ejido. Almería (España)
____
Correspondencia:
José A. Morales-Molina
Hospital de Poniente
(Unidad de Gestión Clínica Interniveles Farmacia Poniente)
04700 El Ejido (Almería)
Correo electrónico: joseantonio.morales@ephpo.es
____
SUMMARY
Objective: We report the clinical management of two cases with probable interaction between ombitasvir/paritaprevir/ritonavir and dasabuvir with oral vitamin K antagonists.
Results: Two Caucasian men were treated with vitamin K antagonists and ombitasvir/paritaprevir/ritonavir and dasabuvir ± ribavirin for 12 and 24 weeks, respectively. Both patients maintained undetectable viral load during the treatment of hepatitis C. After discontinuing ombitasvir/paritaprevir/ritonavir and dasabuvir, a stable and therapeutic INR was achieved. Although for this, it was necessary to increase the dose of oral vitamin K antagonists. However, only after complete antiviral treatment INR values were normalized. Patients presented more than 98% adherence to antiviral and anticoagulant treatment according to direct-counting of medication. They did not present any other pathology that explains the need of VKA dose increase to reach a stable INR in therapeutic range. Recently, an independent and additional effect of ribavirin has been reported in relation to the dose-response decrease of warfarin when combined with the new AAD regimens.
Conclusions: In patients with HCV treated with VKA and DAA, it would be advisable to stop drugs metabolized by CYP2C9. A close clinical monitoring of anticoagulant treatment would be the best recommended therapy. However, additional studies should be performed to determine which the best therapeutic alternative is for these patients.
Key Words: Acenocoumarol, direct acting antivirals, drug interaction, hepatitis C, international normalized ratio, oral anticoagulants, warfarin.
____
BACKGROUND
Ombitasvir/paritaprevir/ritonavir (OBV/PTV/r) and dasabuvir (DSV) are direct-acting antivirals (DAA) indicated in hepatitis C virus (HCV) patients1. On the other hand, acenocoumarol and warfarin have an antagonistic effect on vitamin K. Both drugs are mainly metabolized by cytochrome P450 isoenzymes2. In our patients, an alteration in the anticoagulant effect may increase the risk of haematological disorders. So, oral vitamin K antagonists (VKA) may require more frequent dose adjustment. Currently, there is low evidence of interaction between DAA and VKA2. We report the clinical management of two cases of interaction between OBV/PTV/r and DSV with warfarin and acenocumarol.
CASE 1
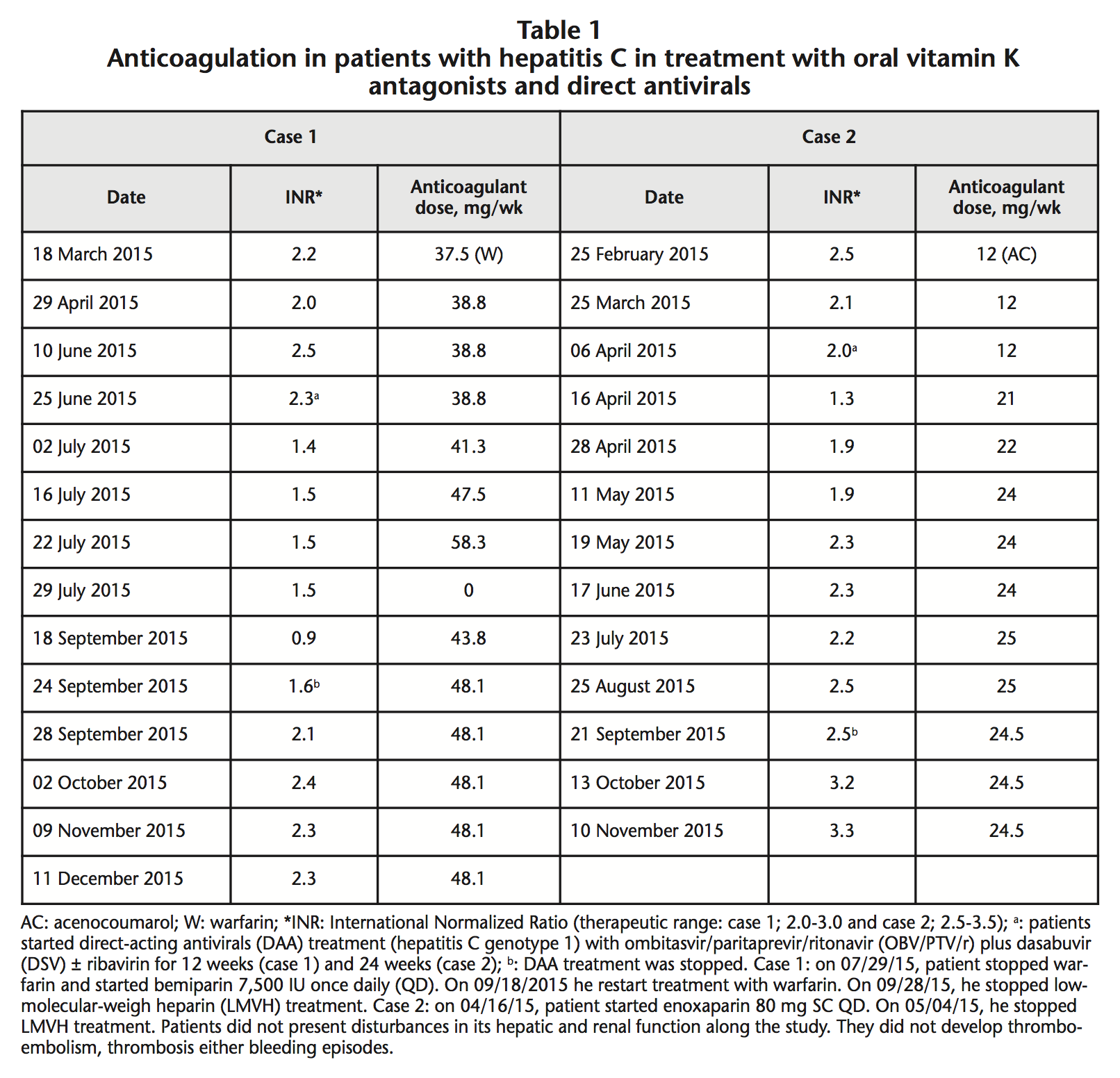
A 37-year-old Caucasian male, was diagnosed in 2003 of HCV-1b and congenital aortic valve stenosis requiring cardiac valve replacement and anticoagulation with warfarin (therapeutic range INR: 2.0-3.0). On May 2015, his basal viral load (VL) was 6,490,000 IU/mL with a Fibroscan of 10.3 kPa. He was classified as Child-Pugh B7 with compensated liver cirrhosis and he was proposed for treatment with DAA. On 06/25/2015 (INR: 2.3; serum albumin: 4.1 g/dL), he started oral treatment with OBV/PTV/r 25 mg/150 mg/100 mg once daily (QD), and DSV 250 mg twice daily (BD) for 12 weeks. On 07/02/2015, his INR decreased to 1.4. On 07/29/2015, the anticoagulant treatment with warfarin was stopped due to failure to reach an INR in therapeutic range. From 07/29/2015 to 09/28/2015, he received treatment with bemiparin 7,500 IU subcutaneous (SC) QD. On 09/24/2015, patient completed HCV treatment with undetectable VL. He achieved sustained viral response 12 weeks later. On 09/18/2015, patient restarted treatment with warfarin 43.8 mg/week. During antiviral therapy (from 06/25/2015 until 09/24/2015), we had to increase dose of warfarin from 38.8 mg/week to 48.1 mg/week (+24%). Following the end of antiviral treatment, we observed a maintained increase of 44% in the INR values. On 09/28/2015, this patient presented stable INR: 2.1, in therapeutic range with warfarin 48.1 mg/week (Table 1).
 He reported no significant changes in his diet or treatment regimen (atenolol 25 mg QD) during the study.
He reported no significant changes in his diet or treatment regimen (atenolol 25 mg QD) during the study.
CASE 2
A 48-year-old Caucasian man was diagnosed in 2005 of abdominal aneurysm, prostate benign hyperplasia and HCV-1a, permanent atrial fibrillation and rheumatic valvulopathy requiring mitral valve replacement and anticoagulation with acenocoumarol (therapeutic range of INR: 2.5-3.5). On March, his baseline VL was 1,270,000 IU/mL with a Fibroscan of 38.7 kPa. He was classified like Child-Pugh B7 with compensated liver cirrhosis. So, he was proposed for treatment with DAA. On 04/06/2015 (INR value: 2.0; Serum albumin: 3.7 g/dL) he started oral treatment with OBV/PTV/r 25 mg/150 mg/100 mg QD plus DSV 250 mg BD and ribavirin 600 mg BD for 24 weeks. On 04/16/2015, his INR decreased (From 2.0 to 1.3). From 04/16/2015 to 05/04/2015, he received enoxaparin 80 mg SC QD. During antiviral therapy, from 04/06/2015 to 05/11/2015, acenocoumarol dose was increased from 12 mg/week to 24 mg/week (+100%). On 09/21/2015 he completed DAA therapy with undetectable VL. Patient achieved sustained VL 12 weeks later. On 10/13/2015, he presented INR: 3.2 (Increase of 30% since the last control). During DAA therapy, he reported no significant changes in his diet or treatment regimen (atenolol 50 mg QD, doxazosin 4 mg QD). Patients had not change in its hepatic and renal function along the study. They not developed thromboembolism, thrombosis, bleeding episodes, alterations in bilirubin values or signs of hemolysis. After performing the probability analysis of pharmacological interactions in the two cases, according to the Horn scale, we obtained a result of 5 points, which indicates a «probable interaction» between the DAA and the VKA3.
DISCUSSION
These clinical cases presented show patients treated with OBV/PTV/r plus DSV and VKA with a INR decrease when they administered together. This decrease disappeared after completing DAA treatment.
Both patients presented a sustained decrease in their INR after starting treatment with DAA and VKA. They required low molecular weight heparin to try to stabilize their INR, but they not achieved therapeutic range.
Only after complete antiviral treatment INR values were normalized. Patients presented more than 98% adherence to antiviral and anticoagulant treatment according to direct-counting of medication. They did not present any other pathology that explains the need of VKA dose increase to reach a stable INR in therapeutic range.
The significant decrease of INR values seems to indicate the implication of several drugs. We could explain this probable interaction by two hypotheses. First, INR values also could have decreased after ritonavir administration through induction of the anticoagulant metabolism of several isoenzymes of CYP450 (CYP1A2, CYP2C9 and CYP2C19)4-6. In addition, the effect of DAA on CYP2C9 should be taken into account. In particular, the DAA are inducers of CYP2C9 cytochrome, by inducing their action, increase the hydroxylation of VKA. After this hydroxylation, VKA are more sensitive to renal elimination, with the consequent decrease in their pharmacological effect in the coagulation cascade7.
Second, a 62% increase in paritaprevir AUC (PTV) has been reported in patients with moderate hepatic impairment. There was also an increase of 950% in the PTV AUC and 325% in the DSV AUC in patients with severe hepatic impairment treated with these drugs8. Both patients had moderate hepatic dysfunction, so an increase of PTV AUC could be expected and it could have led to decrease VKA plasma concentrations. Also, it has been reported that administration of OBV/PTV/r plus DSV does not affect R- and S-warfarin plasma concentrations (≤12% change in AUC) and patients treated with these DAA and VKA did not require VKA doses adjustment9. However, our patients had to increase the dose of VKA to try to achieve the therapeutic range. In a recent study, an independent and additional effect of ribavirin has been reported in relation to the dose-response decrease of warfarin when combined with the new AAD regimens10.
In patients with HCV treated with VKA and DAA, it would be advisable to stop drugs metabolized by CYP2C9. A close clinical monitoring of anticoagulant treatment would be the best recommended therapy. However, additional studies should be performed to determine which the best therapeutic alternative is for these patients.
Conflict of interest: The authors declare no conflicts of interest.
BIBLIOGRAPHY
1. Walker DR, Pedrosa MC, Manthena SR, Patel N, Marx SE. Early View of the Effectiveness of New Direct-Acting Antiviral (DAA) Regimens in Patients with Hepatitis C Virus (HCV). Adv Ther. 2015;32(11):1117-27. doi: 10.1007/s12325-015-0258-5.
2. de Lorenzo-Pinto A, Giménez-Manzorro A, Rodríguez-González CG, Ahumada-Jiménez A, Herranz-Alonso A, Marzal-Alfaro MB, et al. Decreased INR after acenocoumarol ombitasvir/paritaprevir/ritonavir and dasabuvir co-administration. J Clin Pharm Ther. 2016;41(4): 444-46. doi: 10.1111/jcpt.12403.
3. Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother. 2007;41(4):674-80. doi: 10.1345/aph.1H423.
4. Knoell KR, Young TM, Cousins ES. Potential interaction involving warfarin and ritonavir. Ann Pharmacother. 1998;32(12):1299-302. doi: 10.1345/aph.17456.
5. Llibre JM, Romeu J, López E, Sirera G. Severe interaction between ritonavir and acenocoumarol. Ann Pharmacother. 2002;36(4):621-3. doi: 10.1345/aph.19361.
6. Hughes CA, Freitas A, Miedzinski LJ. Interaction between lopinavir/ritonavir and warfarin. CMAJ. 2007; 177(4):357-9. doi: 10.1503/cmaj.061284.
7. Stehle S, Kirchheiner J, Lazar A, Fuhr U. Pharmacogenetics of oral anticoagulants. A basis for Dose Individualization. Clin Pharmacokinet. 2008;47(9):565-94. doi: 10.2165/00003088-200847090-00002.
8. Khatri A, Menon RM, Marbury TC, Lawitz EJ, Podsadecki TJ, Mullally VM, et al. Pharmacokinetics and safety of co-administered paritaprevir plus ritonavir, ombitasvir, and dasabuvir in hepatic impairment. J Hepatol. 2015;63 (4): 805-12. doi: 10.1016/j.jhep.2015.05.029.
9. Menon RM, Badri PS, Wang T, Polepally AR, Zha J, Khatri A, et al. Drug-drug interaction profile of the all-oral anti-hepatitis C virus regimen of paritaprevir/ritonavir, ombitasvir and dasabuvir. J Hepatol. 2015;63(1):20-9. doi: 10.1016/j.jhep.2015.01.026.
10. DeCarolis DD, Westanmo AD, Chen YC, Boese AL, Walquist MA, Rector TS. Evaluation of a Potential Interaction Between New Regimens to Treat Hepatitis C and Warfarin. Ann Pharmacother. 2016 Nov;50(11):909-917. doi: 10.1177/1060028016660325.
____
Download PDF: Anticoagulation in patients with hepatitis C: oral vitamin K antagonists and direct antivirals
