García Basas L1, Sánchez Cuervo M1, Pérez Anchordoqui D2, Alonso Gordoa T3, Álvarez Díaz A1
1 Pharmacy Department. Hospital Universitario Ramón y Cajal. Madrid (Spain)
2 Pharmacy Department. MD Anderson Cancer Center. Madrid (Spain)
3 Oncology Department. Hospital Universitario Ramón y Cajal. Madrid (Spain)
Fecha de recepción: 19/11/2021 – Fecha de aceptación: 14/12/2021
Correspondencia: Lorena García Basas – Pharmacy Department – Onco-Hematology Clinical Trials Unit Hospital Universitario Vall d’Hebron – Passeig de la Vall d’Hebron, 119-129 – 08035 Barcelona (Spain)
lgarciab@vhio.net
____
SUMMARY
Introduction: Clinical trial results with new cancer therapies often differ from the results observed in daily practice. Atezolizumab showed durable responses and a favorable safety profile for patients with advanced urothelial carcinoma refractory to platinum-based chemotherapy in the IMvigor211 trial. This study was conducted to compare patients treated in real clinical practice and IMvigor211 trial regarding baseline characteristics, effectiveness and safety.
Material and methods: Retrospective, observational study of data collection in a tertiary hospital. Patients receiving atezolizumab between January 10, 2018, and March 5, 2020, were included. Primary endpoints were progression free survival, overall survival and the number of discontinuations due to adverse events.
Results: Twenty-nine patients were studied with a median follow up of 5.1 months (range 0-21.6). Median age was 75 years in our cohort and 67 years in the IMvigor211 trial. The median progression free survival was 2.7 months (95%CI 1.9-3.5) versus 2.1 months (95%CI 2.1-2.2) in the IMvigor211. The median overall survival was 5.1 months (95% CI 1.9-8.3) versus 8.6 months (95%CI 7.8-9.6) in the IMvigor211. There was a 13.8% of treatment withdrawals due to immune related toxicity compared to 3.5% of patients who discontinued treatment due to the same cause in IMvigor211.
Conclusion: Patients treated in real clinical practice have less favorable baseline characteristics than patients in the IMvigor211 trial. Results in real clinical practice are similar to those of the clinical trial. Further studies with a longer median follow up and a more significant number of patients would be warranted to confirm these hypotheses.
Key words: Real clinical practice, effectiveness, safety, atezolizumab, urothelial carcinoma, genitourinary oncology.
Atezolizumab en carcinoma urotelial avanzado o metastásico tras quimioterapia a base de platino: experiencia en la práctica clínica real
RESUMEN
Introducción: Los resultados de los ensayos clínicos a menudo difieren de la práctica real. Atezolizumab mostró un perfil de eficacia y seguridad favorable en pacientes con carcinoma urotelial avanzado en recaída tras quimioterapia basada en platino en el ensayo IMvigor211. Este estudio compara las características basales de los pacientes tratados con atezolizumab en la práctica clínica real y en el ensayo IMvigor211 y también su eficacia y seguridad.
Métodos: Estudio observacional retrospectivo realizado en un hospital terciario. Se incluyeron todos los pacientes que recibieron atezolizumab entre el 10 de enero de 2018 y el 5 de marzo de 2020. Se evaluó la supervivencia libre de progresión, la supervivencia global y el número de discontinuaciones por efectos adversos.
Resultados: Se incluyeron 29 pacientes, con un seguimiento de 5,1 meses (rango 0-21,6). La mediana de edad fue 75 años frente a los 67 años de los pacientes del ensayo IMvigor211. La supervivencia libre de progresión fue 2,7 meses (IC95% 1,9-3,5) frente a 2,1 meses (IC95% 2,1-2,2) en el IMvigor211. La supervivencia global fue 5,1 meses (IC95% 1,9-8,3) frente a 8,6 meses (IC95% 7,8-9,6) en el IMvigor211. Hubo un 13,8% de interrupciones por toxicidad frente al 3,5% de pacientes que discontinuaron el tratamiento en el ensayo IMvigor211.
Conclusión: En la práctica clínica real, los pacientes tienen peor estado basal que los del ensayo IMvigor211. Los resultados en la práctica real son similares a los del ensayo clínico. Se necesitan estudios con un mayor seguimiento y número de pacientes para confirmar estas hipótesis.
Palabras clave: Práctica clínica real, eficacia, seguridad, atezolizumab, carcinoma urotelial, oncología genitourinaria.
_____
INTRODUCTION
Clinical trial results with new cancer therapies often differ substantially from the results observed in daily practice. Many factors can influence the outcomes of patients receiving identical interventions in clinical trials and clinical practice1. These factors include the strict inclusion and exclusion criteria, which usually limit the investigational drug use for patients in a better clinical situation than usual2. For most of the new drugs in oncology, this possible uncertainty between clinical trials and the results of actual clinical practice has not been accurately quantified yet, despite its importance and potential impact on the clinical choice3.
Bladder cancer is the second most common urological tumor in Spain after prostate cancer. It is estimated that in 2020, 22,350 new cases of bladder cancer will have been diagnosed in our country, 18,071 of them in men, placing bladder cancer as one of the four most frequently diagnosed tumors in Spain during that year4. The most common histology in bladder cancer is urothelial carcinoma or transitional cell carcinoma5.
Transurethral resection followed by Bacille Calmette Guerin (BCG) instillations or mitomycin C is the standard of care in non-muscle invasive disease, while in muscle invasive disease, it is radical cystectomy together with extended lymphadenectomy, associated with perioperative chemotherapy. Despite cystectomy, approximately 50% of patients with muscle invasive disease will relapse6. Platinum based chemotherapy is the standard of care in previously untreated patients with advanced or metastatic carcinoma and is associated with an overall survival (OS) of approximately 9-15 months7,8. In those patients unfit for cisplatin, carboplatin can be used instead, although carboplatin appears to achieve poorer response rates than cisplatin9,10.
Until the appearance of immunotherapy, the only approved second line chemotherapy agent for the advanced disease was vinflunine, which has an acceptable safety profile11,12. In the last years, PD-1/PD-L1 inhibitors (nivolumab, pembrolizumab, atezolizumab, durvalumab and avelumab) were approved for second line after platinum-based chemotherapy. Recently, avelumab maintenance has shown improved OS compared with best supportive care alone in patients whose disease had not progressed with first line chemotherapy13.
Atezolizumab showed encouraging durable response rates, survival, and tolerability in the phase II non randomized, multicentre single arm study IMvigor210, cohort two, and in the randomized phase III study IMvigor211, which evaluated atezolizumab versus chemotherapy.
OS was the primary endpoint of IMvigor211. OS was tested hierarchically in prespecified populations (stratified by PD-L1 expression) followed by the intention to treat (ITT) population. Atezolizumab was not associated with significantly longer OS than chemotherapy in patients with platinum refractory metastatic urothelial carcinoma overexpressing PD-L1. However, atezolizumab was associated with a longer duration of response than chemotherapy and showed a favorable 12-month OS rate in the ITT analysis14.
The safety profile for atezolizumab in IMvigor211 was favorable compared with chemotherapy, both in patients with high PD-L1 expression as in the ITT population. Fatigue, nausea, constipation, and alopecia were more common in the chemotherapy treated group than in the experimental group. Conversely, atezolizumab treated patients experienced more treatment related pruritus and rash. Additionally, immunotherapy was associated with a lower rate of grade 3/4 adverse events (AEs) (fatigue, anemia, neutropenia, peripheral neuropathy, asthenia, febrile neutropenia, constipation, and ileus) and fewer treatment interruptions14,15.
The role of immunotherapy as a first line treatment in urothelial carcinoma remains doubtful. The only results published are from only two single arm phase 2 monotherapy trials16,17.
In Europe, atezolizumab is approved for platinum treated locally advanced or metastatic urothelial carcinoma since 2017. Subsequently, the drug received funding in Spain. Our centre uses atezolizumab since its approval within the financing conditions18,19.
In this setting, this study was conducted to evaluate similarities and differences between patients treated with atezolizumab in real clinical practice and IMvigor211 trial regarding baseline characteristics, effectiveness, and safety.
The study received approval from Drug Research Ethics Committee in May 2020 (approval letter number 157-20).
MATERIAL AND METHODS
We conducted this retrospective, observational study of data collection in a tertiary hospital. Data were collected from the electronic medical records (HCIS®), the comprehensive drug management system of Pharmacy Service (Hospiwin®), and the off-label use drugs management system (pk_Usos®). All adult patients with advanced or metastatic urothelial carcinoma who received at least one dose of atezolizumab for progression or relapse after platinum-based chemotherapy between January 10, 2018, and March 5, 2020, were included. Patients treated in clinical trial settings were excluded.
Primary endpoints were progression free survival (PFS) (time from treatment initiation to progression of disease or death according to investigator review, whichever occurred first), OS (time from treatment initiation to death from any cause), and the number of discontinuations due to AEs. Secondary endpoints were objective response rate (number of complete and partial responses), duration of response, frequency of severe AEs (classified as grade 3 or 4 based on the Common Terminology Criteria for Adverse Events [CTCAE], from the National Cancer Institute [NCI] version 5.0), time of occurrence of an AE, frequency of dosing delays/discontinuations due to an AE and which one caused them. Progression of disease was verified by RECIST 1.1 2009 criteria.
Patients were followed for safety for 30 days after the last dose (or until initiation of another anticancer therapy if earlier). The same baseline and clinical characteristics as in the IMvigor211 study population were studied. NCCN Clinical Practice Guidelines were used to determine the stage of the disease20.
First line platinum chosen by the oncologist was considered of interest. This variable was not included in the IMvigor211. At the beginning of treatment, the kidney function was also studied since the IMvigor211 excluded patients with calculated creatinine clearance <30 mL/min. These patients are frequent in real clinical practice, as indicated SAUL study21. Creatinine clearance was collected based on the MDRD-4 IDMS equation since it is the centre’s parameter provided by routine biochemistry.
The Kaplan Meier method was used to estimate PFS and OS. ITT population from de IMvigor211 was chosen for the comparative analysis since the patients in our study were not stratified according to PD-L1 expression. Kaplan Meier curves were compared graphically. A subgroup analysis based on the ECOG was performed using the log rank test. Categorical variables were defined by their absolute and relative frequencies, and their 95% confidence interval was estimated using the exact binomial method. Quantitative variables were defined by the mean and the standard deviation, in the case of fulfilling the normality assumption, and by the median and the range in the opposite case. For statistical analysis, IBM® SPSS® Statistics 21.0 software was used.
RESULTS
Baseline characteristics
A total of 29 patients were studied with a median follow up of 5.1 months (range 0-21.6). Comparative baseline characteristics with the IMvigor211 population are shown in table 1. The race was excluded because all the patients were Caucasian. All patients had metastatic disease at the beginning of treatment and had received at least one previous line of treatment in this setting.
Follow up of the efficacy and safety endpoints ended on December 3, 2020. The median age was 75 years (range 60-85), compared with the median age of 67 years in the IMvigor211 (range 33-88). Patients in real clinical practice had a worse baseline performance status than patients in the clinical trial. They also had a higher number of risk factors related to survival and received more previous treatment lines for metastatic disease. The median number of doses received per patient was 3 (range 1-28). 24% of patients would have been excluded from the IMvigor211 trial due to worse performance status (ECOG >1). 65.5% received carboplatin instead of cisplatin, and 10.3% presented a creatinine clearance below 30 mL/min.
Effectiveness
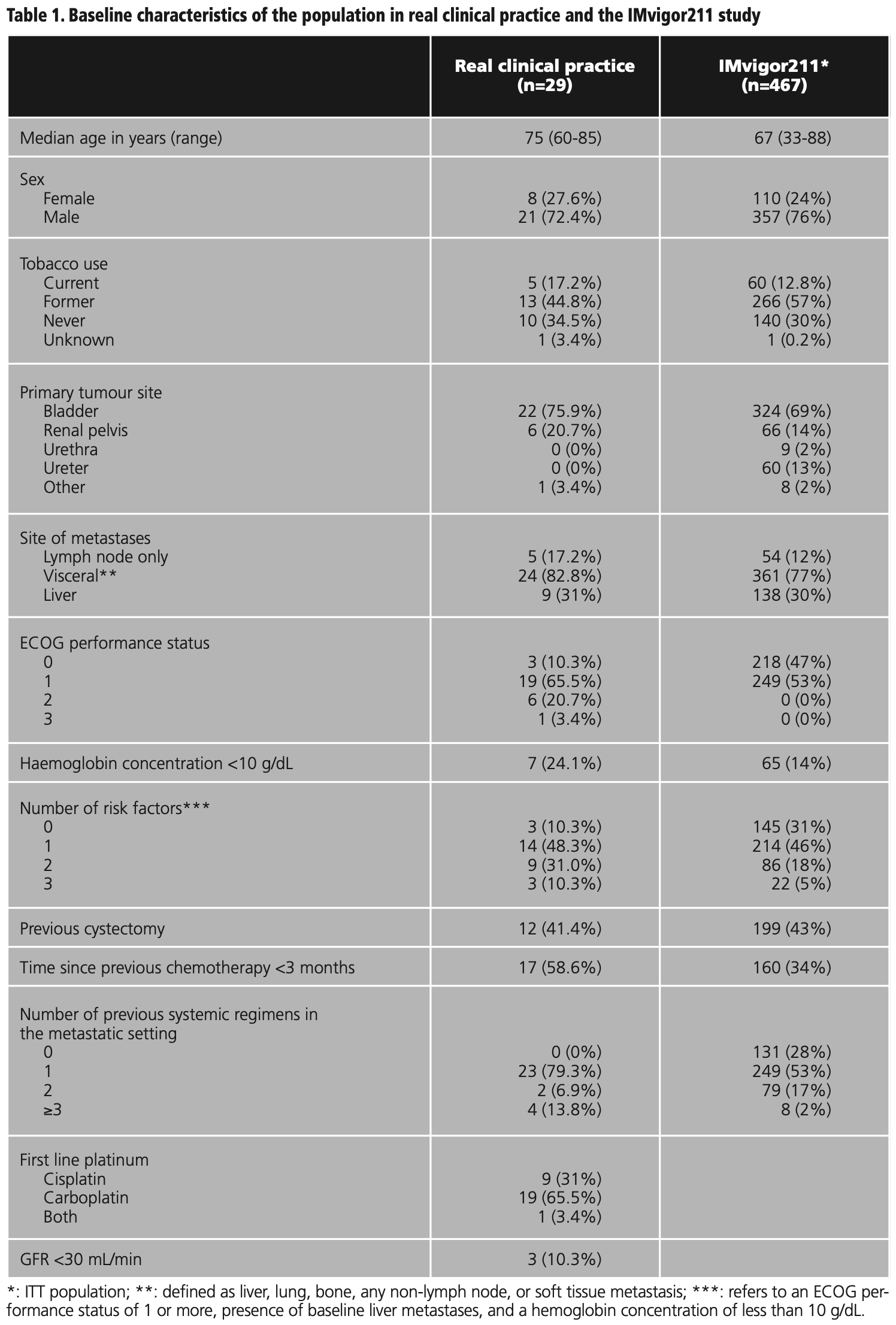
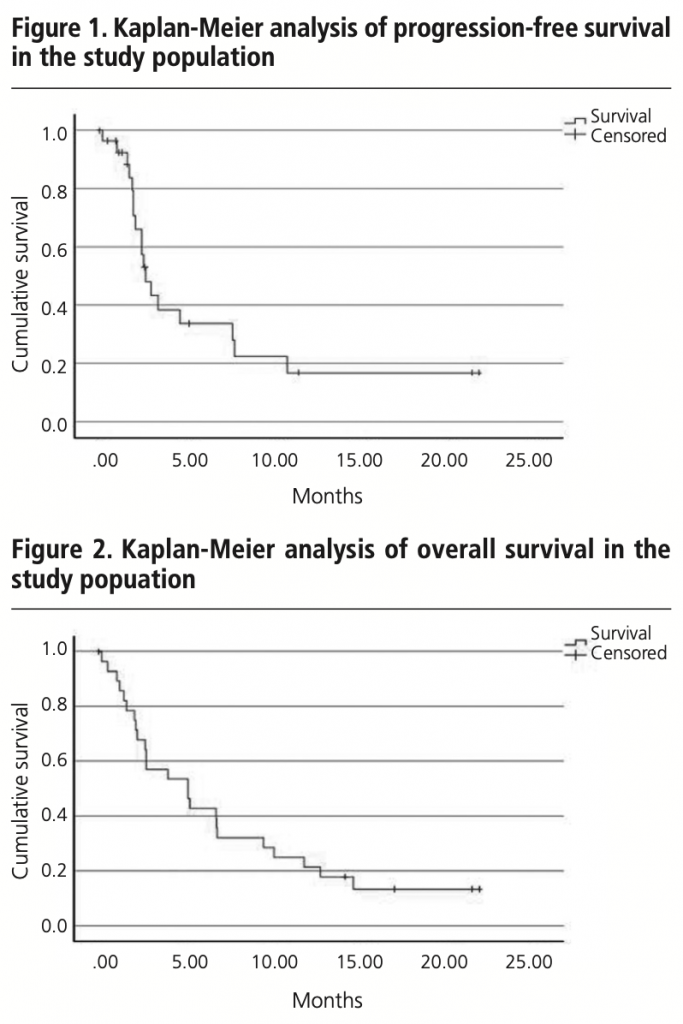
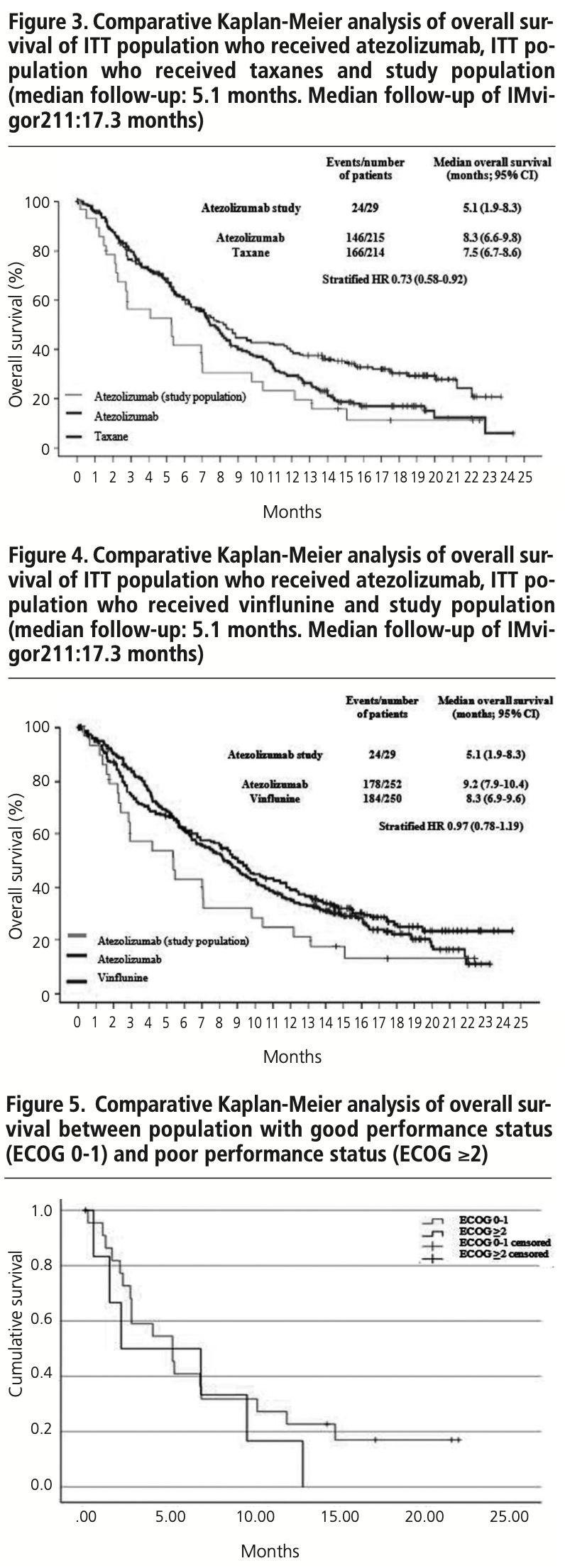
The median PFS in real clinical practice was 2.7 months (95% CI 1.9-3.5) versus 2.1 months (95% CI 2.1-2.2) of PFS in the IMvigor211 (figure 1). The median OS was 5.1 months (95% CI 1.9-8.3) versus 8.6 months (95% CI 7.8-9.6) in the IMvigor211 (figure 2). Kaplan Meier curves of OS of the study population and ITT population from IMvigor211 were compared graphically (both versus taxanes and vinflunine, figures 3 and 4). The OS rate at 12 months was 20.7%. Secondary efficacy endpoints are listed in table 2.
Patients who would have been excluded from the IMvigor211 due to ECOG ≥2 (24.1%) had shorter OS than patients with ECOG 0-1 (2.1 months (95% CI 0-8.4) versus 5.1 months (95% CI 2.2-7.9), log-rank test p=0.399) (figure 5).
Safety
Seven (24.1%) patients experienced at least one severe AE. Anorexia (5, 17.2%) and fatigue (5, 17.2%) were the most observed severe AEs followed by anaemia (2, 6.9%), constipation (2, 6.9%), dyspnoea (1, 3.4%), ileus (1, 3.4%) and neutropenia (1, 3.4%). The median onset of severe AEs was 2.1 months. Secondary safety endpoints are listed in table 3.
There were four (13.8%) treatment withdrawals due to possible treatment related toxicity compared to 3.5% of patients who discontinued treatment due to the same cause in IMvigor211. Immune mediated renal toxicity (2, 6.9%), neutropenia (1, 3.4%), and liver toxicity (1, 3.4%) were the AEs that led to treatment discontinuations. Five patients (17.2%) delayed the administration of atezolizumab due to an AE. Among the reasons for the delays in treatment were liver enzyme alteration (2, 6.9%), asthenia (1, 3.4%), dyspepsia (1, 3.4%), and acute renal failure (1, 3.4%).
One (3.4%) death occurred, while four (1%) deaths occurred in IMvigor211.
DISCUSSION
Compared to IMvigor211, the patients in real clinical practice were older, with a higher number of prognostic risk factors, and had received more prior treatment lines in the metastatic setting. Nearly half of the IMvigor211 patients had an ECOG of 0 compared to 10% of the patients treated at our centre. Furthermore, 65.5% of patients in real clinical practice received carboplatin compared to 31% of patients who received cisplatin. Considering that carboplatin is only used for frail patients, this is another data that supports these worse baseline characteristics.
Despite the data being insufficient to compare the survival observed in this study and the survival of IMvigor211 by using the log rank test, results suggest that the effectiveness of atezolizumab in real clinical practice is similar to that seen in the clinical trial. Median PFS was 2.7 months, while in IMvigor211, the median PFS was 2.1 months. The difference in OS (5.1 months versus 8.6 months) could be attributed to the shorter follow up time and the worse baseline characteristics of the study population. These results are in line with those of other real-life studies with a similar number of patients22,23: Serrano Giménez et al. show a PFS of 4.0 months and an OS of 6.0 months in a study with 38 patients. Ballesta López et al. show a PFS of 4.8 months and an OS of 10.2 months in a study with 21 patients, although this population had lower median age, and 95% had ECOG 0-1.
Although statistical significance was not met, the difference in OS between patients with ECOG 0-1 and patients who would have been excluded from IMvigor211 because of ECOG ≥2 was distinctive. As in the previous studies1, we can attribute this difference to the worst baseline conditions, frequently seen in real clinical practice. In the metastatic setting, Necchi et al. found an OS more than two times lower among patients with ECOG ≥2 than those with ECOG of 024.
The AEs profile was similar between this study and the IMvigor211, although it is difficult to differentiate whether anorexia or fatigue is attributable to treatment or the disease itself. The high proportion of patients who discontinued because of possible toxicity associated with atezolizumab compared to the reference study (13.8% versus 3.5%) is remarkable. In other real-life studies, the percentage of severe AEs is slightly lower (7.9%)22.
Within real life studies with atezolizumab, the SAUL trial deserves special mentioning. In this phase IIIb trial, Sternberg et al. evaluated atezolizumab in patients with ECOG greater than 1, impaired renal function (below 30 mL/min but above 15 mL/min), autoimmune disease, symptomatic brain metastases, and nonurothelial histology. All this population would have been excluded in IMvigor211 but are frequent in clinical practice25. In this population, atezolizumab also proved to be an effective and well tolerated treatment even in complex patients with comorbidities. Its tolerability in patients with ECOG 2 was equivalent to tolerability in the “IMvigor211 like” more positively selected population. The final results will be known in 2022. If the results of this study are compared with those of the SAUL trial, it is also observed that the population starts treatment with worse baseline characteristics (they are older and have worse ECOG). This could be one factor that justifies the worse efficacy results (OS of 5.1 months versus 8.7 months in the SAUL trial) and safety (13.8% of discontinuations due to AEs versus 8%).
These demonstrated differences between real life populations and clinical trials highlights the importance of real life, phase IIIb and IV pharmacovigilance trials to expand evidence in populations more representative of the real one in clinical practice.
As a strength of this study, all patients were evaluated by the same oncologist’s team, which provides uniformity in evaluating the toxicities they experienced. The retrospective nature, the difference in sample size compared to the IMvigor211 study, and the limited follow up period are some of its limitations.
CONCLUSIONS
The following conclusions can be drawn from the present study: firstly, patients treated in real clinical practice have less favorable baseline characteristics than patients in the IMvigor211 trial. Secondly, results in the real clinical practice of atezolizumab in advanced or metastatic urothelial cancer after platinum-based chemotherapy are similar to those of the clinical trial that granted PD-1/PD-L1 inhibitors the indication in this context. Further studies in real clinical practice with a longer median follow up and a more significant number of patients would be warranted to confirm these hypotheses.
Conflict of interests: The authors declare that they do not present a conflict of interest.
BIBLIOGRAPHY
1. Templeton AJ, Vera-Badillo FE, Wang L, et al. Translating clinical trials to clinical practice: outcomes of men with metastatic castration-resistant prostate cancer treated with docetaxel and prednisone in and out of clinical trials. Ann Oncol. 2013;24(12):2972-7. DOI: 10.1093/annonc/mdt397.
2. George SL. Reducing patient eligibility criteria in cancer clinical trials. J Clin Oncol. 1996;14(4):1364-70. DOI: 10.1200/JCO.1996.14.4.1364.
3. Bui TBV, Burgers DM, Agterof MJ, van de Garde EM. Real-world effectiveness of palbociclib versus clinical trial results in patients with advanced/metastatic breast cancer that progressed on previous endocrine therapy. Breast Cancer Basic Clin Res. 2019;13:117822341882323. DOI: 10.1177/1178223418823238.
4. Cancer numbers in Spain. Spanish Society of Medical Oncology (SEOM). 2018 https://seom.org/seomcms/images/stories/recursos/Las_Cifras_del_cancer_en_Espana2018.pdf Accessed March 12 2020.
5. Veskimäe E, Espinos EL, Bruins HM, et al. What is the prognostic and clinical importance of urothelial and nonurothelial histological variants of bladder cancer in predicting oncological outcomes in patients with muscle-invasive and metastatic bladder cancer? A European Association of Urology muscle-invasive and metastatic bladder cancer guidelines panel systematic review. Eur Urol Oncol. 2019;2(6):625-42. DOI: 10.1016/j.euo.2019.09.003.
6. Witjes JA, Bruins HM, Cathomas R, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur Urol 2020;S030228382030230X. DOI: 10.1016/j.eururo.2020.03.055.
7. Von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602-8. DOI: 10.1200/JCO.2005.07.757.
8. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC Study 30986. J Clin Oncol. 2012;30(2):191-9. DOI: 10.1200/JCO.2011.37.3571.
9. Witjes JA, Babjuk M, Bellmunt J, et al. EAU-ESMO consensus statements on the management of advanced and variant bladder cancer-An international collaborative multistakeholder effort†. Eur Urol. 2020;77(2):223-50. DOI: 10.1016/j.eururo.2019.09.035.
10. Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol Off J Eur Soc Med Oncol. 2012;23(2):406-10. DOI: 10.1093/annonc/mdr156.
11. Bellmunt J, Théodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-61. DOI: 10.1200/JCO.2008.20.5534.
12. Javlor® (vinflunine) Injection prescribing information: Boulogne (France); Pierre Fabre Médicament; 2014.
13. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-30. DOI: 10.1056/NEJMoa2002788.
14. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. The Lancet. 2018;391(10122):748-57. DOI: 10.1016/S0140-6736(17)33297-X.
15. Spanish Society of Medical Oncology (SEOM). Tecentriq® (Atezolizumab) (Atezolizumab) for metastatic urothelial carcinoma in individuals who previously received platinum chemotherapy or who are ineligible for cisplatin. SEOM drug evaluation report; 2018. https://seom.org/seomcms/images/stories/Informes_SEOM/IEV_atezolizumab_vejiga.pdf. Accessed March 20 202014.
16. O’Donnell PH, Plimack ER, Bellmunt J, et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: Results of a phase IB study. J Clin Oncol. 2015;33(7_suppl):296-296. DOI: 10.1200/jco.2015.33.7
17. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017;389(10064):67-76. DOI: 10.1016/S0140-6736(16)32455-2.
18. Information search engine on the financing situation of medicines (BIFIMED) July 2020. https://www.mscbs.gob.es/profesionales/medicamentos.do?metodo=verDetalle&cn=719470. Accessed July 21 2020.
19. Valencia Soto C, Flores Moreno S, Ventayol Bosch P. Pembrolizumab/atezolizumab in unresectable or metastatic locally advanced urothelial carcinoma in adults after previous platinum-based therapy or in patients not candidates for cisplatin. Report of the GENESIS-SEFH Group. http://gruposdetrabajo.sefh.es/genesis/genesis/Enlaces/InformesHosp_abc.htm. Accessed July 22 2020.
20. National Comprehensive Cancer Network. Bladder Cancer (Version 5.2017). https://jnccn.org/view/journals/jnccn/15/10/article-p1240.xml?ArticleBodyColorStyles=pdf-5590 Accessed June 19 2020.
21. Sternberg CN, Loriot Y, James N, Choy E, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol. 2019;76(1):73-81. DOI: 10.1016/j.eururo.2019.03.015.
22. Serrano Giménez R, Muñoz Burgos M, Cordero Ramos J, Artacho Criado S, Galván Banqueri M, Márquez Saavedra E. Real-life study of atezolizumab in second-line urothelial carcinoma. National Congress of the Spanish Society of Hospital Pharmacy (SEFH) 2020.
23. Ballesta López O, Beltrán García I, Centelles Oria M, et al. Effectiveness and safety of atezolizumab in metastatic urothelial carcinoma: a real-life study. Congress of the Spanish Society of Hospital Pharmacy (SEFH) 2020.
24. Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: Retrospective international study of invasive/advanced cancer of the urothelium (RISC). Eur Urol. 2017;71(2):281-9. DOI: 10.1016/j.eururo.2016.09.042.
25. Bambury RM, Benjamin DJ, Chaim JL, et al. The safety and efficacy of single‐agent pemetrexed in platinum‐resistant advanced urothelial carcinoma: A large single‐institution experience. The oncologist 2015;20(5):508-15. DOI: 10.1634/theoncologist.2014-0354.
_____