Claramunt García R1, Muñoz Cid CL2, Sánchez Ruiz A3.
- Hospital Pharmacy Specialist. Pharmacy Department. Hospital Virgen de Altagracia. Manzanares, Spain.
- Hospital Pharmacy Specialist. Pharmacy Department. Hospital de la Serranía de Ronda. Ronda, Spain.
- Hospital Pharmacy Specialist. Pharmacy Department. Hospital Universitario de Jaén, Spain.
Fecha de recepción: 21/02/2023 – Fecha de aceptación: 21/03/2023
Correspondencia: Raquel Claramunt García · Hospital Virgen de Altagracia de Manzanares, Avda Don Emiliano García Roldán s/n C.P.13200, Manzanares, Ciudad Real, España · raquel_kudt93@hotmail.com
____
Summary
Purpose: To establish, through an indirect comparison (IC) against placebo, whether abrocitinib, baricitinib and upadacitinib can be considered equivalent alternatives in efficacy for the treatment of atopic dermatitis.
Methods: A Pubmed search was performed for pivotal clinical trials (CTs) of abrocitinib, baricitinib, and upadacitinib for atopic dermatitis, as monotherapy (MT) and in combination with topical corticosteroids (TC). The main variable for comparison was EASI75 (Eczema Area and Severity Index) at week 16 after start of treatment. Relative risk (RR) compared to placebo was calculated. Finally, an IC of these drugs was performed using the Bucher method (ITC calculator, Indirect Treatment Comparisons, of the Canadian Agency for Health Technology Assessment). Results were analyzed, seeing if there were statistically significant differences between these three drugs.
Results: Eight CT were found: two of abrocitinib (1 TC and 1 MT), three of baricitinib (1 TC and 2 MT) and three of upadacitinib (1 TC and 2 MT). All of them versus placebo as common comparator. None of the IC showed statistically significant differences, except in the comparison of baricitinib 2mg versus upadacitinib 15mg, and in baricitinb 4mg versus upadacitinib 30mg, in all cases in favor of upadacitinib.
Conclusion: Given that no statistically significant differences have been established between the different drugs in terms of efficacy, and in those in which it has been found we cannot know if it is a clinically relevant difference, the choice of one or the other should be based on safety and efficiency criteria.
Keywords: abrocitinib, baricitinib, upadacitinib, atopic dermatitis, efficacy.
Eficacia comparativa entre abrocitinib, baricitinib y upadacitinib en el tratamiento de la dermatitis atópica
Objetivo: Establecer, mediante una comparación indirecta (CI) frente a placebo, si abrocitinib, baricitinib y upadacitinib pueden considerarse alternativas equivalentes en eficacia para el tratamiento de la dermatitis atópica.
Métodos: Se realizó una búsqueda en Pubmed de ensayos clínicos pivotales (EC) de abrocitinib, baricitinib y upadacitinib para la dermatitis atópica, como monoterapia (MT) y en combinación con corticosteroides tópicos (CT). La variable principal de comparación fue el EASI75 (Eczema Area and Severity Index) en la semana 16 tras el inicio del tratamiento. Se calculó el riesgo relativo (RR) en comparación con placebo. Se realizó un CI de estos fármacos mediante el método Bucher (calculadora ITC, Indirect Treatment Comparisons, de la Agencia Canadiense de Evaluación de Tecnologías Sanitarias). Se analizó si existían diferencias estadísticamente significativas entre ellos.
Resultado: Se encontraron ocho EC: dos de abrocitinib (1 CT y 1 MT), tres de baricitinib (1 CT y 2 MT) y tres de upadacitinib (1 CT y 2 MT). Todos ellos frente a placebo como comparador común. Ninguno de las CI mostró diferencias estadísticamente significativas, excepto en la comparación de baricitinib 2mg vs upadacitinib 15mg, y en baricitinb 4mg vs upadacitinib 30mg, a favor de upadacitinib.
Conclusiones: Dado que no se han establecido diferencias estadísticamente significativas entre los distintos fármacos en términos de eficacia, y en aquellos en los que sí se han encontrado no podemos saber si es una diferencia clínicamente relevante, la elección de uno u otro debería basarse en criterios de seguridad y eficiencia.
Palabras clave: abrocitinib, baricitinib, upadacitinib, dermatitis atópica, eficacia.
____
Introduction
Atopic dermatitis is an inflammatory skin disease, which usually presents with sensitive and dry skin, localized or disseminated eczematous lesions, generally accompanied by a strong itching sensation.1
The scarce information on the incidence and prevalence of this pathology could be due to the wide interindividual variability, the different definitions of the disease, the diagnostic criteria and the lack of a universally accepted index for classifying the severity of the disease, among others2. Atopic dermatitis is more common in children than in adults, and in overcrowded urban areas3. Regarding adults, a study published in 20184 reported a prevalence of atopic dermatitis in adults in the EU of 4.4 % (95 % CI: 4.2-4.6).
Optimal treatment of atopic dermatitis requires an approach that addresses different aspects, such as elimination of factors that trigger disease flares (heat, low humidity, anxiety, stress, skin infections, contact with allergens), restoration of skin barrier function and skin hydration, patient education and pharmacological treatment5. Measures such as moisturizing the skin well, the use of topical treatments, phototherapy and oral antihistamines are usually sufficient to control mild disease6.
From the results obtained from different investigations over the years, it has been evidenced that the Janus kinase (JAK)/Signal Transducers and Activators of Transcription (STAT) pathway is one of the essential signaling pathways in various inflammatory diseases, such as atopic dermatitis. In addition, some interleukins such as IL-4, IL-13 and IL-31 have been found to be major contributors to the chronic pruritus of this disease, and these are transmitted through the JAK-STAT pathway. Therefore, it appears that JAK inhibitors could be promising candidates for the treatment of severe atopic dermatitis7.
Several JAK inhibitor drugs have been approved in recent years for the treatment of severe atopic dermatitis, including baricitinib, upadacitinib and abrocitinib. However, clinical trials comparing all these alternatives with each other are not available to date. Therefore, the aim of this study is to establish, through an indirect comparison (IC) versus placebo, whether abrocitinib, baricitinib and upadacitinib can be considered equivalent alternatives in efficacy for the treatment of atopic dermatitis, when used both in monotherapy (MT) and concomitantly with topical corticosteroids (TC).
Methods
A Pubmed search was conducted for pivotal clinical trials (CTs) of abrocitinib (200mg/24h, 100mg/24h), baricitinib (2mg/24h, 4mg/24h) and upadacitinib (15mg/24h, 30mg/24h) for atopic dermatitis, both in monotherapy and in combination with topical corticosteroids. The keywords used were: atopic dermatitis, abrocitinib, baricitinib, upadacitinib. The main characteristics of each trial were collected (randomization, arms of each trial, diagnosis, number of patients, mean age, EASI (Eczema Area and Severity Index) prior to treatment initiation) and assessed for important differences between them.
The EASI75 results at week 16 after treatment initiation were used as the primary endpoint for comparison. With the EASI75 results (in %), the relative risk (RR) with respect to placebo was calculated. Finally, with these values, an IC of these drugs was performed using the Bucher method (ITC calculator, Indirect Treatment Comparisons, of the Canadian Agency for Health Technology Assessment). The lower doses of the 3 drugs (abrocitinib 100mg, baricitinib 2mg, upadacitinib 15mg) and the higher doses (abrocitinib 200mg, baricitinib 4mg, upadacitinib 30mg) were compared with each other, both in monotherapy and in combination with topical corticosteroids. The results were analyzed to see if there were statistically significant differences between these three drugs.
Results
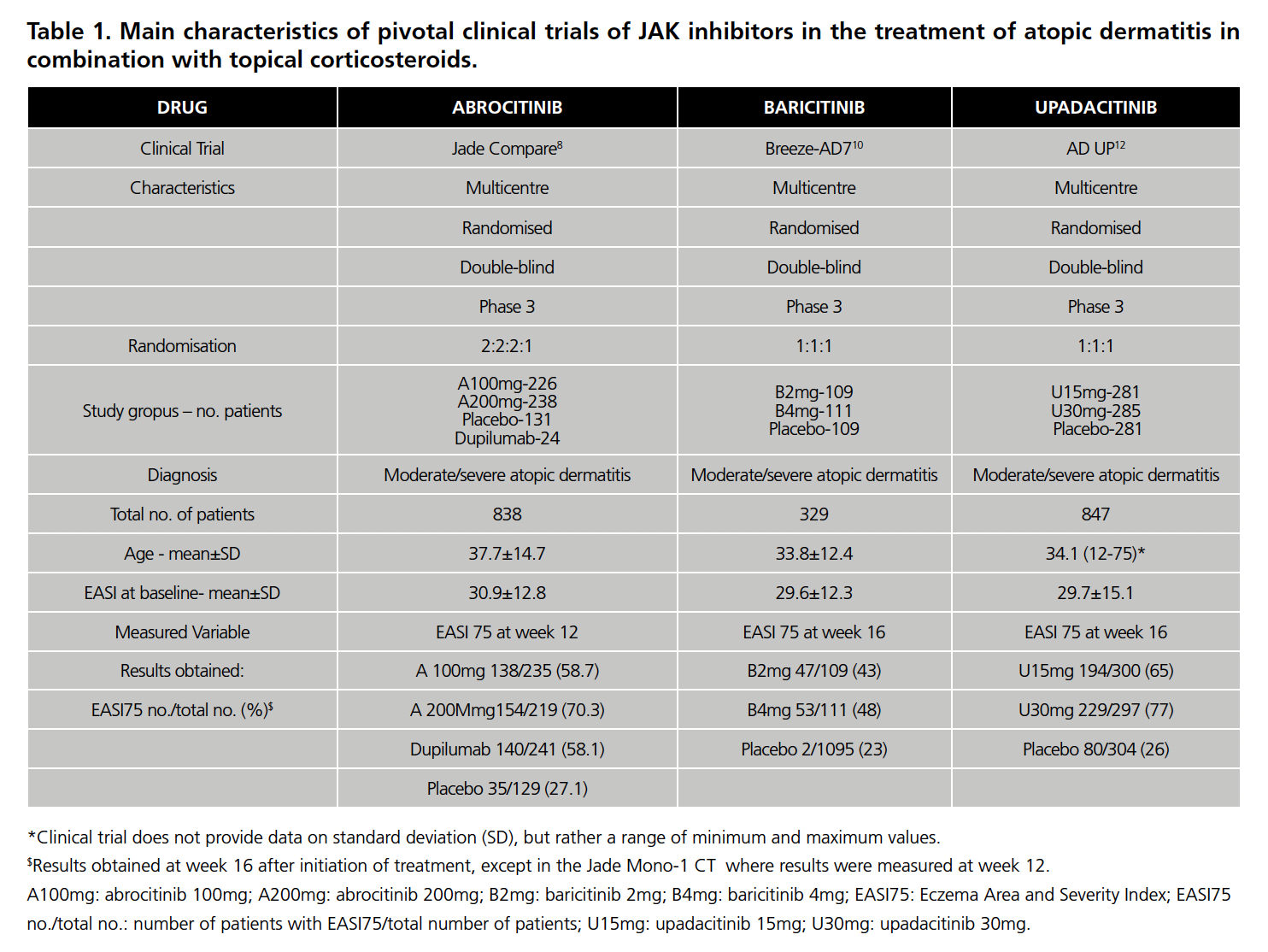
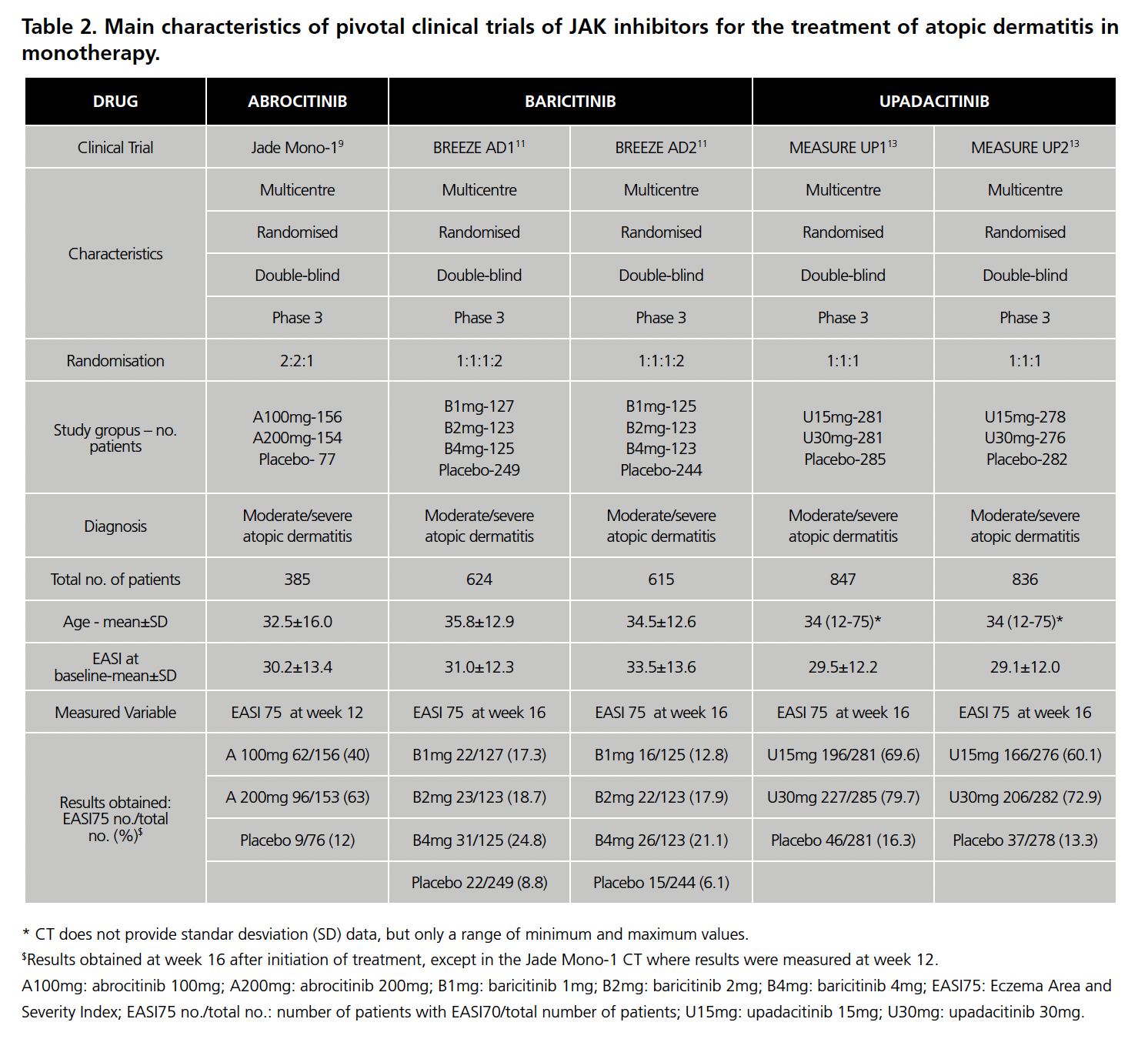
Eight CTs were found: 2 of abrocitinib8,9 (1 with TC and 1 in MT), 3 of baricitinib10,11 (1 with TC and 2 in MT) and 3 of upadacitinib12,13 (1 with TC and 2 in MT). All of them versus placebo as a common comparator. The main characteristics of all the CEs are summarized in Table 1 (with TC) and Table 2 (in MT).
Regarding CTs in combination with TC, all studies presented a similar methodology (design, characteristics of the study population, inclusion criteria, primary endpoint). However, the pivotal trial of upadacitinib included patients younger than 18 years of age (corresponding to 12% of the total), unlike the other CTs only included population of legal age. In addition, the sample size differs between CTs (the sample size of the upadacitinib CT is approximately three times larger than that of baricitinib, while that of abrocitinib is in an intermediate position between the other two).
As for the CTs in MT, they also presented similar methodology. However, the abrocitinib CT was the only one not to include patients under 18 years of age (13.5% of patients in the upadacitinib CT and 22% in the baricitinib CT). Regarding the use of TC in these CTs, the abrocitinib CT specifies that no rescue medication or topical corticosteroids were allowed and that those who used them were excluded. The baricitinib CT performs a separate analysis of the patients who did not require rescue with TC, which are shown in Table 1, and which were used to calculate the RR and, therefore, the IC. The CT for upadacitinib points out that patients who used TC were considered non-responders.
In both situations (MT and TC), in the abrocitinib CTs the EASI75 is measured at 12 weeks from the start of treatment, while in the others at week 16.
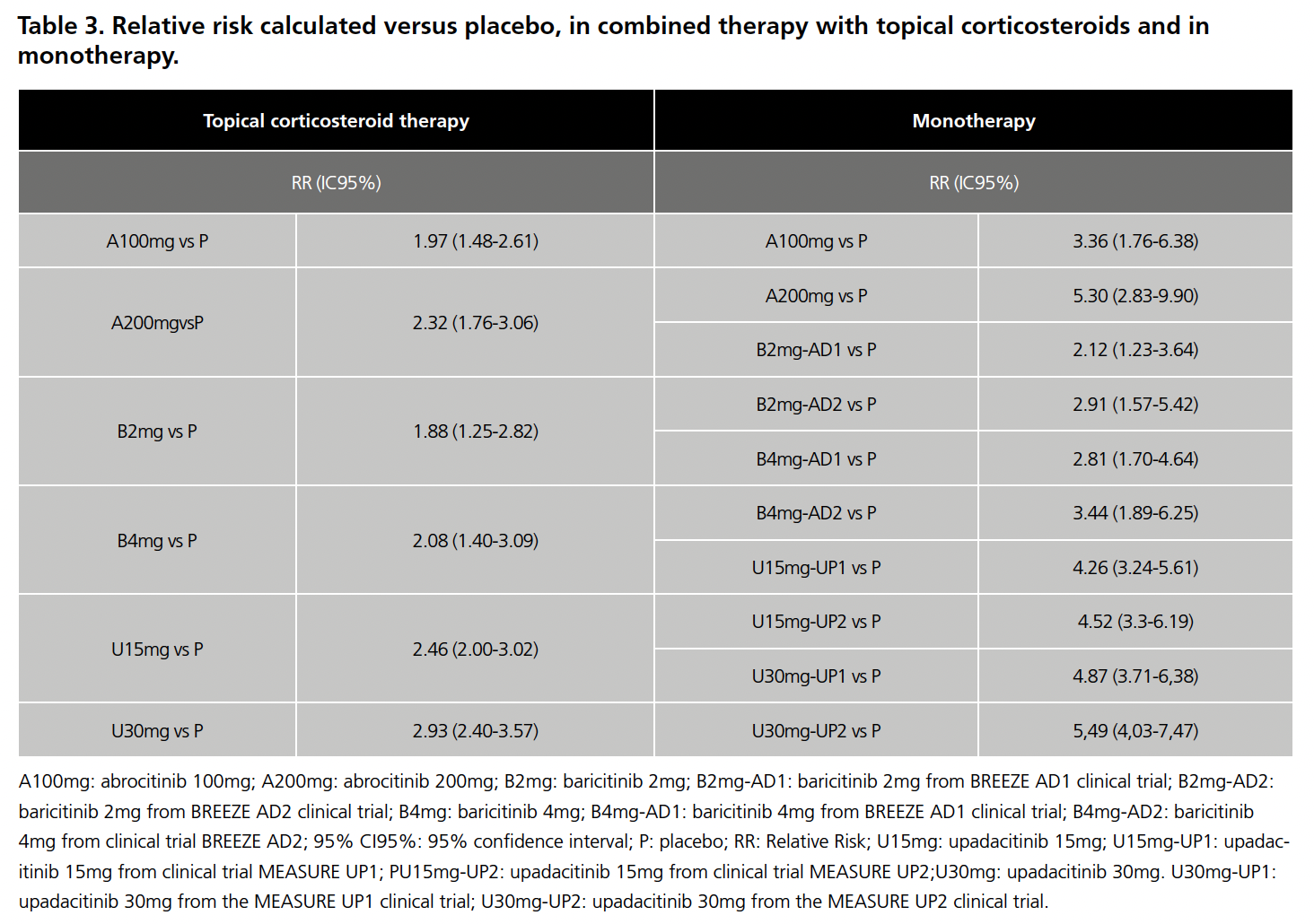
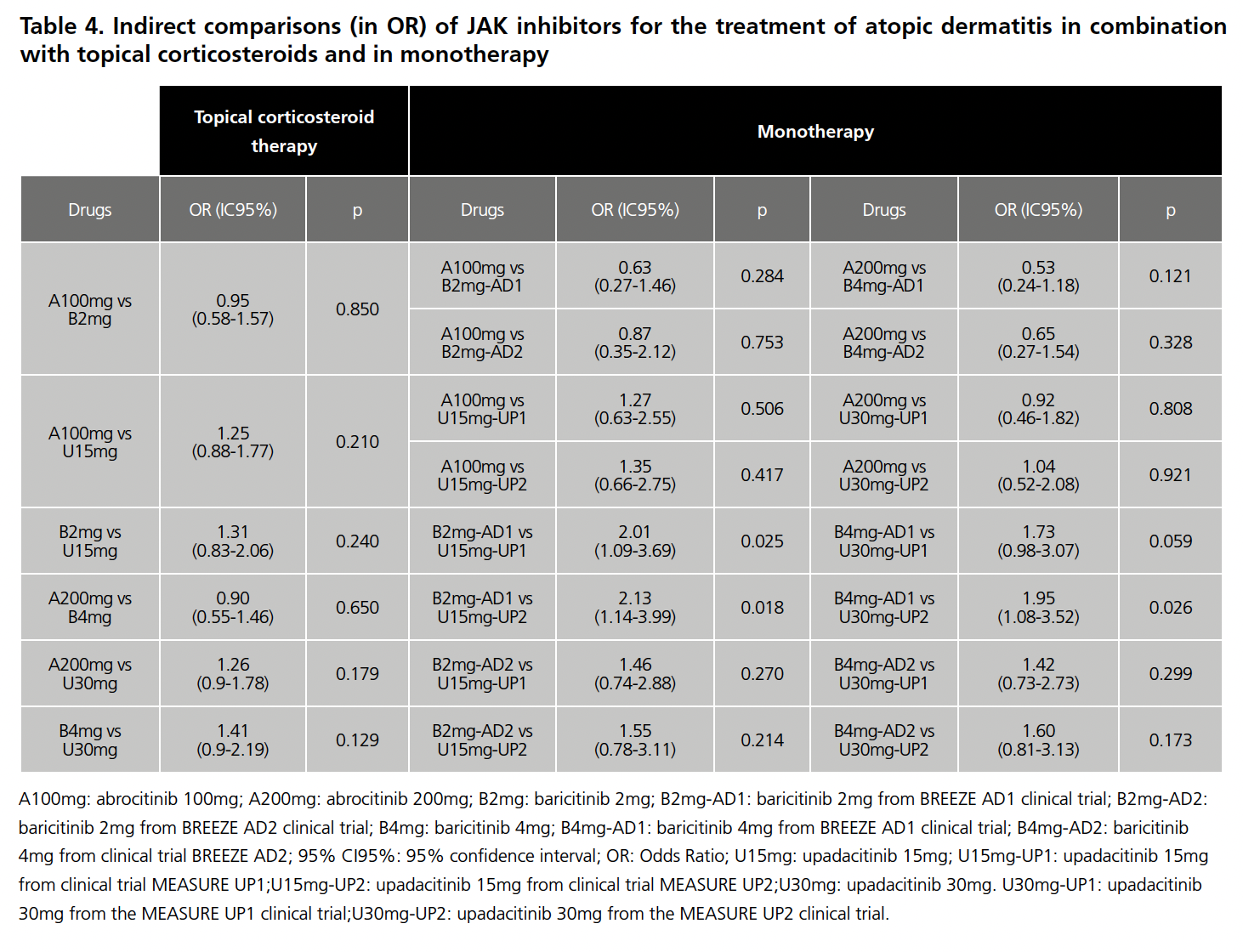
Once the limitations for both scenarios had been analyzed, they were accepted for IC and the RR was calculated with respect to placebo for each drug in the 8 CTs, the results of which are summarized in Table 3. The data for baricitinib 1mg were excluded from the analysis because this presentation is not commercialized in Spain, as were those for dupilumab in the Jade Compare trial because it is not a JAK inhibitor. With these RR values, and after applying the Butcher method, the Odds Ratio (OR) values were obtained, which are summarized in Table 4.
None of the results of the indirect comparisons showed statistically significant differences, except in the comparison of baricitinib 2mg (CT BREEZE AD1) versus upadacitinib 15mg (CT MEASURE UP1 AND UP2), and baricitinb 4mg (CT BREEZE AD 1) versus upadacitinib 30mg (CT MEASURE UP2), in all three cases in favor of upadacitinib.
Conclusions
According to the results obtained in combination therapy with TC, given that no statistically significant differences have been established between the different drugs in terms of efficacy, the choice of one or the other for the treatment of atopic dermatitis should be based on safety and efficiency criteria.
Regarding the results obtained in the ICs of the CTs in MT, it could be that baricitinib had a more modest benefit than upadacitinib. However, of the 8 CTs performed between these two drugs, only 3 showed a statistically significant difference, and therefore it would also be necessary to assess whether this difference is clinically relevant. This, together with the limitations described with respect to the differences between the pivotal CTs, means that these results should be taken with caution and the drug should be individually adapted to each patient according to the interaction profile, adverse effects and cost.
For all these reasons, it would be of particular interest to have a direct comparison of these drugs to confirm equivalence.
The authors declare no conflict of interest
Bibliography
- Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nature. Jan 22. Vol 21; 21-40.
- Raimondo A, Lembo S. Atopic Dermatitis: Epidemiology and Clinical Phenotypes. Pract Concept. 2021;11(4):e2021146. DOI: https://doi.org/10.5826/dpc.1104a146
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(1):8–16. DOI: 10.1159/000370220. PMID: 25925336
- Barbarot S, Auziere S, Gadkari A, et al. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73(6):1284-1293. DOI: 10.1111/all.13401. PMID: 29319189.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014 Dec;134(6):e1735-44. doi: 10.1542/peds.2014-2812. PMID: 25422009.
- Ng SY, Begum S, Chong SY. Does Order of Application of Emollient and Topical Corticosteroids Make a Difference in the Severity of Atopic Eczema in Children? Pediatr Dermatol. 2016 Mar-Apr;33(2):160-4. doi: 10.1111/pde.12758. Epub 2016 Feb 9. PMID: 26856694.
- Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: Emerging topical and oral Janus kinase inhibitors. Allergol Int. 2022 Jan;71(1):40-46. doi: 10.1016/j.alit.2021.10.004. Epub 2021 Nov 21. PMID: 34815171.
- Bieber T, Simpson EL, Silverberg JI, et. al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N Engl J Med. 2021 Mar 25;384(12):1101-1112. doi: 10.1056/NEJMoa2019380. PMID: 33761207.
- Simpson EL, Sinclair R, Forman S, et. al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020 Jul 25;396(10246):255-266. doi: 10.1016/S0140-6736(20)30732-7. PMID: 32711801.
- Reich K, Kabashima K, Peris K, et. al. Efficacy and Safety of Baricitinib Combined With Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2020 Dec 1;156(12):1333-1343. doi: 10.1001/jamadermatol.2020.3260.
- Simpson EL, Lacour JP, Spelman L, et. al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020 Aug;183(2):242-255. doi: 10.1111/bjd.18898.
- Reich K, Teixeira HD, de Bruin-Weller M, et. al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021 Jun 5;397(10290):2169-2181. doi: 10.1016/S0140-6736(21)00589-4.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et. al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021 Jun 5;397(10290):2151-2168. doi: 10.1016/S0140-6736(21)00588-2.
____