Giménez Giner S, Llopis Alemany A, Porta Oltra B, Climente Martí M
Hospital Universitario Doctor Peset. Servicio de Farmacia. Valencia (España)
Fecha de recepción: 14/02/2022 – Fecha de aceptación: 05/03/2022
Correspondencia: Sara Giménez Giner – Hospital Universitario Doctor Peset (Servicio de Farmacia) – Avenida Gaspar Aguilar, 90 – 46017 Valencia (España)
saragimenezginer@gmail.com
____
Summary
Autoimmune hemolytic anemia (AIHA) is disorder that involves the destruction of erythrocytes, differentiating between warm AIHA (WAIHA) with extravascular hemolysis mediated by IgG, cold agglutinin disease (CAD) with intravascular hemolysis mediated by IgM/Cd3 or mixed AIHA. Treatment of WAIHA consists of corticosteroids, immunoglobulins, rituximab, immunosuppressants, cyclophosphamide and splenectomy, whereas in CAD the response to corticosteroids is low.
The 46-year-old patient came to the emergency department with a drop of 4.5 hemoglobin points in 15 days, associated with tachycardia, muco-cutaneous pallor and dyspnea. After analytical parameters compatible with AIHA and Coombs test Cd3+/IgM-, a diagnosis of CAD was made and rituximab and warm transfusion support were started. When intravascular haemolysis intensified, intravenous immunoglobulin, plasmapheresis and darbepoetin alpha were associated with no clinical improvement, later receiving bortezomib as third line and eculizumab due to refractoriness. The Coombs’ test was repeated, with a result compatible with WAIHA and slight splenomegaly associated due to extravascular hemolysis, so a splenectomy was performed achieving stabilization of analytical parameters and allowing the patient to be discharged.
This clinical case evidences a conversion in the etiology of AIHA, with initial diagnosis of CAD since Coombs’ test was Cd3+/IgG- and converted to WAIHA after 37 days of admission since Coombs’ test changed to Cd3-/IgG+.
Key words: Autoimmune hemolytic anemia, autoantibody, hemoglobin, rituximab, eculizumab.
Conversión de etiología de anemia hemolítica autoinmune
Resumen
La anemia hemolítica autoinmune (AIHA) es una patología que conlleva la destrucción de eritrocitos, diferenciando entre AIHA por anticuerpos calientes (WAIHA) con hemólisis extravascular por IgG, AIHA por crioaglutininas (CAD) con hemólisis intravascular por IgM/Cd3 o AIHA mixtas. El tratamiento de AIHA por anticuerpos calientes consiste en corticoides, inmunoglobulinas, rituximab, inmunosupresores, ciclofosfamida y esplenectomía, mientras que en AIHA por crioaglutininas la respuesta a corticoides es baja.
La paciente de 46 años acudió a urgencias con caída de 4,5 puntos de hemoglobina en 15 días, asociando taquicardia, palidez y disnea. Tras parámetros analíticos compatibles con AIHA y test de Coombs Cd3+/IgM- se diagnosticó de CAD e inició rituximab y transfusiones con calentador. Ante una intensificación de la hemólisis intravascular se asoció inmunoglobulina intravenosa, plasmaféresis y darbepoetina alfa sin mejoría clínica, recibiendo posteriormente bortezomib como tercera línea y eculizumab ante refractariedad. Se repitió el test de Coombs evidenciando en ese momento un diagnóstico por WAIHA, con ligera esplenomegalia asociada a la hemólisis extravascular, por lo que se realizó una esplenectomía alcanzando una estabilización de los parámetros analíticos y permitiendo el alta de la paciente.
Este caso clínico evidencia una conversión en la etiología de la AIHA, con diagnóstico inicial de CAD ante test de Coombs Cd3+/IgG- y cambio a WAIHA después de 37 días de ingreso ante Coombs Cd3-/IgG+.
Palabras clave: Anemia hemolítica autoinmune, autoanticuerpo, hemoglobin, rituximab, eculizumab.
_____
Introduction
Autoimmune hemolytic anemia (AIHA) is a type of anemia produced by autoantibodies that react against erythrocyte antigens, leading to their destruction. Depending on the optimal reaction temperature of the autoantibodies, it is classified in warm AIHA (WAIHA; 37ºC), mediated by IgG with predominant extravascular hemolysis; cold agglutinin disease (CAD; 4ºC), mediated by IgM and Cd3 with predominant intravascular hemolysis; and mixed AIHA with both antibodies1.
Physical examination usually reveals muco-cutaneous paleness and jaundice, and is accompanied by decreased hemoglobin (Hb), increased total serum bilirubin (BST), increased lactate dehydrogenase (LDH), and absence of haptoglobin1.
The initial treatment of WAIHA consists of corticosteroids with the possibility of association with intravenous immunoglobulins or plasmapheresis, resorting to off-label rituximab, immunosuppressants or cyclophosphamide boluses and splenectomy in case of refractoriness. In CAD, the response to corticosteroids is low and rituximab is started in the first line1.
Case description
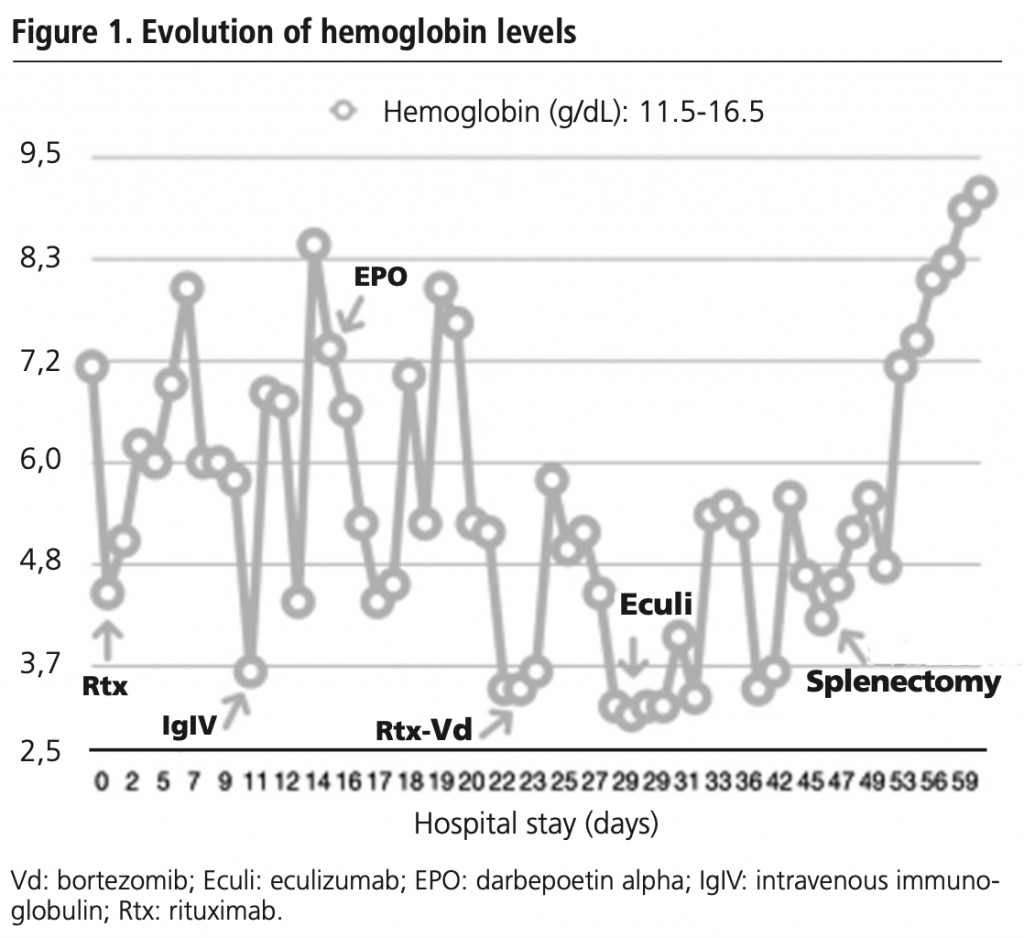
A 46-year-old female kidney transplant recipient came to the emergency department for haemolytic anaemia study after a drop of 4.5 Hb points in 15 days. She presented with an initial picture of regular tachycardia, muco-cutaneous pallor, asthenia, dyspnea of two weeks’ evolution and hypotension. Laboratory tests showed Hb 7.1 g/dl, BST 2.4 mg/dl, LDH 546 IU/l and undetectable haptoglobin.
After a positive Coombs’ test with a positive monospecific test for Cd3 and negative for IgG compatible with a diagnosis of CAD, off-label rituximab 375 mg/m2/week was started as first line with warm transfusion support. When intravascular haemolysis intensified, it was combined with intravenous immunoglobulin (1 g/kg/day for 2 days), daily plasmapheresis with in-line blood warmer and darbepoetin alpha 150 mcg once weekly. After several days without clinical improvement, third line with off-label bortezomib (1.3 mg/m2 days 1, 4, 8 and 11 of a 21-day treatment cycle) was initiated and darbepoetin alpha was increased to 300 mcg once weekly. However, episodes of very severe refractory intravascular haemolysis continued and a fourth line of eculizumab 900 mg once weekly was initiated.
Given the therapeutic failure and evolution of AIHA to Hb 3.4 g/dl, BST 4.35 mg/dl and LDH 509 IUI/l, Coombs’ test was repeated, with a positive monospecific test for panagglutinin IgG and cryoagglutinin not significant, with a result compatible with WAIHA. At this point, transfusion support was adjusted for at room temperature transfusion (with no warmer) and a thoracic-abdominal CT scan was performed, which detected slight splenomegaly associated with extravascular haemolysis processes, so a splenectomy was performed on the patient.
After surgery, there was a clear decrease in BST (1.47 mg/dl) and LDH (454 IUI/l) but not in Hb (4.8 g/dl), and a second cycle of immunoglobulins (1 g/kg/day for 5 days) was required to show a clear improvement in the analytical parameters (Hb 7.1 g/dl, BST 0.56 mg/dl). Twelve days after surgery, the patient was discharged with a diagnosis of autoimmune haemolytic anaemia due to warm antibodies and an analytical stabilization of the parameters at Hb 9.1 g/dl, BST 0.60 mg/dl and LDH 391 IU/l. The evolution of hemoglobin levels is shown in figure 1.
Discussion
AIHA is a type of acquired anaemia with a low incidence of 1-3 cases per 100,000 patients/year. Diagnosis by immunohaematology will be fundamental, as it allows differentiation between AIHA due to warm antibodies or cryoagglutinins and thus the required supportive treatment or the possibility of response to corticosteroids in the first line2.
Scientific evidence on the best therapeutic approach is limited and without approved therapeutic indication (off-label), as there are clinical consensus guidelines but they are based on clinical case reports. Rituximab is the most studied and effective treatment for AIHA regardless of the mechanism of haemolysis, accompanied by splenectomy in case of extravascular haemolysis. The use of oral immunosuppressants (mycophenolate mofetil and sirolimus), cyclophosphamide boluses or azathioprine has been relegated to a third line since the introduction of rituximab in the treatment of AIHA3. Bortezomib has been postulated as a treatment in subsequent therapeutic lines given its interference with the antigen-antibody presentation process, with evidence of clinical cases in which it achieves stabilization of analytical parameters, but not always a disappearance of the antibody title4. Finally, eculizumab has also made inroads in the treatment of cryoagglutinin or mixed AIHA, given its similar pathophysiological mechanism to the intravascular haemolysis that occurs in paroxysmal nocturnal haemoglobinuria or haemolytic uraemic syndrome for which it has an approved indication. As a consequence of its effect on terminal complement, eculizumab has demonstrated an improvement in haemolytic parameters in several clinical cases5-7, a benefit that was also significant in the phase 2 DECADE trial8.
In the case of our patient, the initial diagnosis was CAD given the positive Coombs’ test with a positive monospecific test for Cd3 and negative for IgG; but after 37 days of admission, a conversion in the etiology of WAIHA was evidenced by repeating the positive Coombs’ test, this time with a positive monospecific test for panagglutinin IgG and non-significant cryoagglutinin. Currently, no history of a change in the etiology of AIHA has been reported in the literature.
Another peculiarity of the case is the patient’s history of renal transplantation and the maintenance of tacrolimus blood concentration in therapeutic range, given the intense changes in the patient’s haematocrit and the extensive binding of the immunosuppressant to erythrocytes, which leads to a lower concentration of the drug, but with proportionally higher activity as the free fraction increases9. This highlights the need to increase the frequency of pharmacokinetic monitoring in patients with moderate-to-severe anaemia, as well as the need to adjust the therapeutic range of the immunosuppressant in patients with intense haematocrit changes10.
Conflict of interests: The authors declare that they do not present a conflict of interest.
BIBLIOGRAPHY
1. Liebman, HA. Autoimmune Hemolytic Anemia. Med Clin North Am. 2017 Mar;101(2):351-359.
2. Hill, QA. The diagnosis and management of primary autoimmune haemolytic anaemia. Br J Haematol. 2017 Feb;176(3):395-411.
3. Reynaud, Q. Efficacy and safety of rituximab in auto-immune hemolytic anemia: A meta-analysis of 21 studies. Autoimmun Rev. 2015 Apr;14(4):304-13.
4. Muhsen, IN. Bortezomib for immune thrombocytopenia and autoimmune hemolytic anemia. Hematol Oncol Stem Cell Ther. 2019 Jun 10:S1658-3876 (19)30051-2.
5. Röth, A. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009 Apr 16;113(16):3885-6.
6. Gupta, N. Long-term response of refractory primary cold agglutinin disease to eculizumab therapy. May 2013; Annals of Hematology 93(2).
7. Shapiro, R. Eculizumab as a bridge to immunosuppressive therapy in severe cold agglutinin disease of anti-Pr specificity. Clin Case Rep. 2015 Nov;3(11):942-4.
8. Röth, A. Eculizumab in cold agglutinin disease (DECADE): an open-label, prospective, bicentric, nonrandomized phase 2 trial. Blood Adv. 2018 Oct 9; 2(19): 2543-2549.
9. Prograf®. Ficha Técnica del medicamento. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS).https://cima.aemps.es/cima/pdfs/ es/ft/61006/FT_61006.pdf.
10. Sikma, MA. Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation. Clin Pharmacokinet. 2020 Jun;59(6)-771-780.
____