Maza-Larrea JA1, Gutiérrez-Villegas I2, Rosado-Hernández F3, Peredo-Gómez KM2, Puerto-García M4, Pérez-Barragán A5, Quiroz-Martínez A6, Berrios-Bárcenas EA7, Rojas-Velasco G8
1 Departamento de Farmacología Clínica. Instituto Nacional de Cardiología. Ciudad de México (México)
2 Unidad de Seguimiento Farmacoterapéutico. Instituto Nacional de Cardiología. Ciudad de México (México)
3 Centro Institucional de Farmacovigilancia. Instituto Nacional de Cardiología. Ciudad de México (México)
4 Universidad West-Hill. Facultad de Medicina. Ciudad de México (México)
5 Universidad La Salle. Facultad de Ciencias Químicas. Ciudad de México (México)
6 Departamento de Cardiología 8° Piso del Instituto Nacional de Cardiología Chávez. Ciudad de México (México)
7 Departamento de Consulta Externa del Instituto Nacional de Cardiología Chávez. Ciudad de México (México)
8 Unidad de Terapia Intensiva del Instituto Nacional de Cardiología Ignacio Chávez. Ciudad de México (México)
Fecha de recepción: 21/01/2022 – Fecha de aceptación: 28/02/2022
Correspondencia: Med/Q.F.B. José Antonio Maza Larrea – Departamento de Farmacología Clínica del Instituto Nacional de Cardiología Ignacio Chávez – Juan Badiano No. 01. Col. Selección XVI, Alcaldía Tlalpan – 14080, Zona de Hospitales Tlalpan, Ciudad de México (México)
jose.maza@cardiologia.org.mx
____
SUMMARY
The use of oral and intravenous corticosteroids as a treatment for SARS-CoV-2 infection has been shown to inhibit the exaggerated inflammatory response, reducing symptoms and days of hospitalization of patients. However, its use is controversial because not enough clinical studies have been made to verify the safety of the drugs.
Objective: To assess the safety profile of corticosteroids treatment, at high and low doses, in suspected or confirmed patients with COVID-19, determining the most frequent side effects in patients, and assessing whether the administration of the drugs represents a greater benefit than the risk of presenting these effects.
Methods: Ambispective study of active pharmacovigilance at a cohort of confirmed or suspected COVID-19 patients, treated with intravenous and oral corticosteroids. 366 patients were evaluated and divided into 3 groups: use of methylprednisolone (155 mg average) every 24 hours for 3 days, dexamethasone (6 mg) every 24 hours for 10 days, and a control group.
Results: The distribution of the cases with hyperglycemia was 33 in high doses and 82 with low doses of corticosteroids and both high and low doses have a similar distribution in cases of infections. When evaluating the harshness and severity of hyperglycemia in the two groups with corticosteroids, it is observed that patients with high doses present more harsh (48%). In case of harshness and severity of infections it is observed that patients with high doses present more harsh (62%) and more severe (79%) cases than those who were administered low doses.
Key words: COVID-19, SARS-CoV-2, corticosteroids, dexamethasone, methylprednisolone, side effects, antibiotics, hyperglycemia, infections.
Seguridad de corticoesteroides en pacientes con COVID-19: un estudio de farmacovigilancia
RESUMEN
El uso de corticoides orales e intravenosos como tratamiento para la infección por SARS-CoV-2 ha demostrado inhibir la respuesta inflamatoria exagerada, reduciendo los síntomas y los días de hospitalización de los pacientes. Sin embargo, su uso es controvertido porque no se han realizado suficientes estudios clínicos para verificar la seguridad de los medicamentos.
Objetivo: Evaluar el perfil de seguridad del tratamiento con corticoides, a dosis altas y bajas, en pacientes con sospecha o confirmación de COVID-19, determinando los efectos secundarios más frecuentes en los pacientes, y valorando si la administración de los fármacos representa un mayor beneficio que el riesgo de presentar estos efectos.
Métodos: Estudio ambispectivo de farmacovigilancia activa en una cohorte de pacientes confirmados o sospechosos de COVID-19, tratados con corticoides intravenosos y orales. Se evaluaron 366 pacientes y se dividieron en 3 grupos: uso de metilprednisolona (155 mg promedio) cada 24 horas por 3 días, dexametasona (6 mg) cada 24 horas por 10 días y un grupo control.
Resultados: La distribución de los casos con hiperglucemia fue de 33 casos usando dosis altas y 82 con dosis bajas de corticoides, tanto las dosis altas como las bajas tienen la misma distribución en los casos de infecciones. Al evaluar la severidad y gravedad de la hiperglucemia en los dos grupos con corticoides, se observa que los pacientes con dosis altas presentan mayor gravedad (48%). En caso de severidad y gravedad de las infecciones se observa que los pacientes con dosis altas presentan casos más graves (62%) y más severos (79%) que los que recibieron dosis bajas.
Palabras clave: COVID-19, SARS-CoV-2, corticoides, dexametasona, metilprednisolona, efectos secundarios, antibióticos, hiperglucemia, infecciones.
____
INTRODUCTION
In December 2019, in Wuhan, China, a public health crisis occurred, which spread and caused great global repercussions. COVID-19 has infected millions of people and a high percentage of them haven’t survived1,2.
An exaggerated systemic inflammation produced by the disease is the cause that leads to serious complications in patients, for this reason, doctors have resorted to treatments that avoid the response of pro-inflammatory cells, which show great efficacy, and at the same time, that have been shown to reduce mortality. Although different treatment regimens have been used for COVID-19, no specific treatment for the disease has been established4,5.
The use of oral and intravenous corticosteroids as a treatment for SARS-CoV-2 infection has been shown to inhibit the exaggerated inflammatory response, reducing symptoms and days of hospitalization of patients. However, its use is controversial because not enough clinical studies have been made to verify the safety of the drugs.
By the end of 2020, 412 cases of suspected side effects to drugs used as treatment for COVID-19 infection were notified to the Spanish Agency for Medicines and Health Products (SAMHP), of which 83% were considered serious, 22 of the cases were related to glucocorticoids, and 8 of them were due to dexamethasone. 40% of these side effects represented gastrointestinal and hepatobiliary abnormalities7.
Since there is not enough information on the use of corticosteroids in patients with COVID-19, it is essential to develop a pharmacovigilance study to determine the risk-benefit balance of corticosteroids as a treatment for the disease, and to determine the most frequent side effects after the administration of high and low doses of corticosteroids.
BACKGROUND
COVID-19 is characterized for producing respiratory symptoms ranging from an ordinary cough to severe acute respiratory syndrome16.
Coronaviruses are unicaternary RNA viruses that belong to the coronaviridae family. They have a spherical shape from which small projections surround them and contain the Spike protein. Once infected by the virus, the human body reacts by perpetuating a systemic inflammatory response, which begins with the production of pro-inflammatory molecules, called cytokines, such as interleukins (IL) 1β, 6 and 8, and tumor necrosis factor (TNF). A poorly regulated inflammatory response, as occurs in patients with severe infection, produces the accumulation of pro-inflammatory cells (monocytes and macrophages) in the lung tissue, resulting in pulmonary damage3,4.
The main site of infection for COVID-19 is the respiratory system. Subunits 1 and 2 of the Spike proteins interact with angiotensin 2 (ACE2) receptors, located in the bronchial tree and alveoli. The binding of the virus-receptor is the entrance gate of the infection to the alveolar cells, where the replication and propagation begins17.
There are different tools to establish the diagnosis of COVID-19. These tests are capable of detecting viral nucleic acids, antigens, antibodies, among others. The results of each test depend on the stage of the disease.
For the detection of viral nucleic acids, the nasopharyngeal swab thru reverse-transcriptase polymerase chain reaction (RT-PCR) test is used. This test should be performed once symptoms have begun, or after at least 5 days after the patient’s exposure to the virus to reduce the possibility of a false negative result. The immunoassay serologic test detects IgG and IgM antibodies for COVID-19; however, the use of this test is not reliable for detecting acute infections because the immune response and detectable levels of immunoglobulins occur approximately 15 to 20 days after symptoms18.
To date, there is no specific antiviral treatment for COVID-19 infection. Currently, treatment is mainly based on symptom control and oxygen therapy to maintain adequate oxygen saturation in patients who do not require hospitalization. Patients with moderate to severe symptoms should be hospitalized, and those with acute respiratory failure require intubation and treatment in an intensive care unit.
Among the most commonly used medications in hospitalized patients are corticosteroids, which are used primarily to decrease the excessive inflammatory response. In addition, antiviral agents (lopinavir/ritonavir, remdesvir) and immunomodulators (hydroxychloroquine, tocilizumab), among others have been used. The use of anticoagulants, have been used to reduce the risk of thromboembolism19.
The use of corticosteroids is indicated in patients with COVID-19 due to their ability to reduce the inflammatory response produced by the disease. The RECOVERY study from the University of Oxford showed a decrease of 1/3 in the mortality of intubated patients. The study verified the efficacy of low-dose intravenous and oral dexamethasone treatment (6 mg/day for 10 days) in randomized hospitalized patients with COVID-19 and severe disease criteria. They concluded that the treatment reduced mortality to 28 days in mechanically ventilated patients8.
In the case of methylprednisolone, in the study conducted at Stony Brook University Hospital, New York, 153 patients diagnosed with COVID-19 by RT-PCR were evaluated under supplemental oxygen requirements. Each patient received a medium dose of 160 mg for 5 to 10 days. The most frequently reported side effects in these patients were bacteremia, in-hospital pneumonia, and gastrointestinal bleeding. Among the results, it could be observed that the treatment reduced the required days of ventilatory assistance, therefore, days of hospitalization22.
According to the Pharmacovigilance Program of the Hospital Universitari de Bellvitge, corticosteroids were the drugs with the most side effects reported; infections being the most frequently, followed by complications in diabetic patients and gastrointestinal bleeding20.
The World Health Organization (WHO) carried out a systematic review of the side effects presented in patients treated with COVID-19, and reported that serious corticoesteroids-related side effects occurred with high-dose administration, infections being the most frequent. On the other hand, under lower doses, patient mortality was related to specific causes such as mechanical ventilation and cardiac arrhythmias, but not side effects21.
Pharmacovigilance is important to asses the side effects presented after the administration of drugs; therefore, active pharmacovigilance protocols are useful to stablish the safety of a treatment. In this study, under a method of direct identification of side effects with the execution of multipurpose databases, laboratory studies and changes in the behavior of diseases were recorded after the administration of corticosteroids treatment.
It is important to remember that corticosteroids administered after short periods, present like immunosuppression, associated infections, hyperglycemia and osteoporosis9,10.
Hyperglycemia is one of the most common side effects in SARS-CoV-2 patients treated with corticosteroids because they inhibit gluconeogenesis, decrease pancreatic insulin production, and promote lipolysis in adipose tissue. In patients with a history of diabetes, these effects are perpetuated, leading to severe hyperglycemia11.
In addition, the pancreas also has the ability to release steroids during an infectious process, therefore, the use of corticosteroids in diabetic patients under COVID-19 infections represents a risk of hyperglycemia. It is of great importance to carry out a thorough review where it is possible to verify the safety of the use of corticosteroids as a treatment for the disease12,13.
PROBLEM STATEMENT
What is the safety profile of methylprednisolone and dexamethasone in suspected and confirmed COVID-19 patients under treatment?
Currently, there is no specific treatment established worldwide for SARS-CoV-2 disease, the treatments used decrease the symptoms and speed up the recovery process, however, they do not represent a cure for the disease. These treatment plans have been used in Mexico and have shown favorable results. At the Ignacio Chavez National Institute of Cardiology, methylprednisolone was used as the initial treatment. Later, the use of low-dose dexamethasone was implemented, based on the RECOVERY study. RECOVERY results demonstrated that dexamethasone reduced endothelial dysfunction and prothrombotic effects, thus reducing symptoms, days of hospitalization and mortality in patients under mechanical ventilation.
HYPOTHESIS
The increase of basal glucose levels and the risk of a hospital acquired infection are related to the dose of administration of corticosteroids such as methylprednisolone and dexamethasone at high and low doses respectively in suspected and confirmed patients with COVID-19.
JUSTIFICATION
As there is no specific treatment for SARS-CoV-2 disease, it is important to ensure that the treatment regimens used are safe. In addition, they must be effective for the patient and guarantee that the benefit after administration is greater than the risk of presenting side effects and their complications.
OBJECTIVE
To assess the safety profile of corticosteroids treatment, at high and low doses, in suspected or confirmed patients with COVID-19 at the Ignacio Chávez National Institute of Cardiology, determining the most frequent side effects in patients, and assessing whether the administration of the drugs represents a greater benefit than the risk of presenting these effects.
METHOD
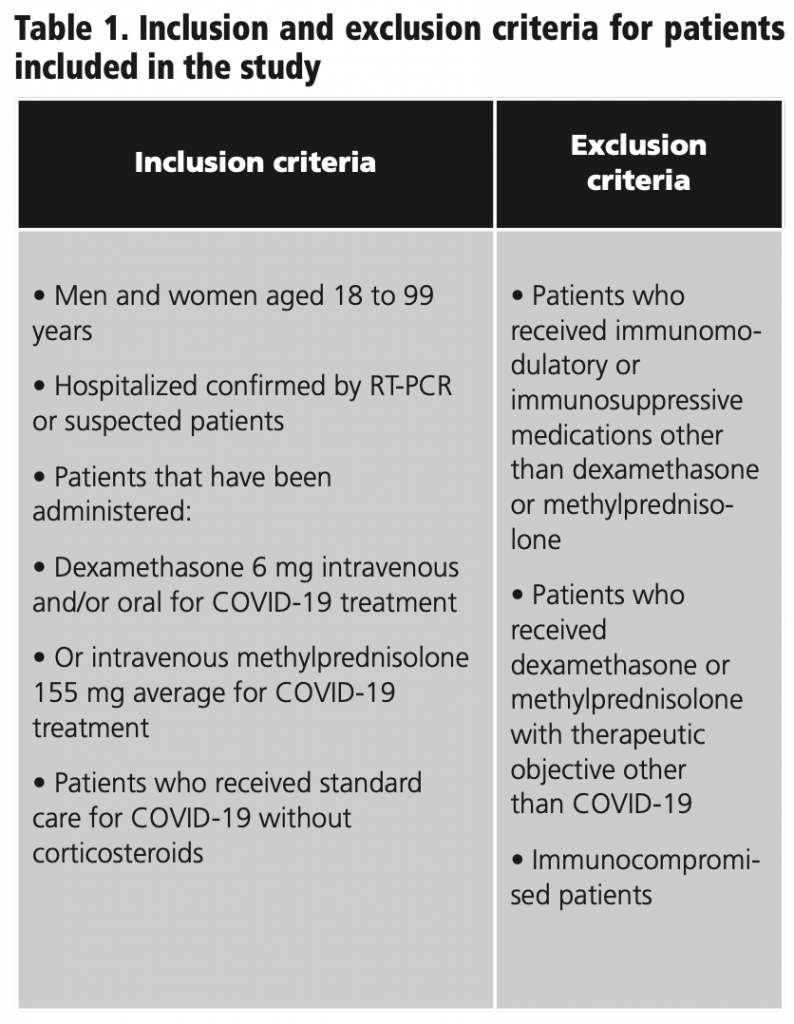
Ambispective study of active pharmacovigilance at a cohort of confirmed or suspected COVID-19 patients hospitalized at the Ignacio Chávez National Institute of Cardiology, from April 11 to December 31, 2020, treated with intravenous and oral corticosteroids. 366 patients were evaluated and divided into 3 groups: use of methylprednisolone (155 mg average) every 24 hours for 3 days, dexamethasone (6 mg) every 24 hours for 10 days, and a control group of patients with no use of corticosteroids (image 1). 87 patients in the study were excluded for meeting exclusion criteria (table 1).
Evaluation of corticosteroid-associated hyperglycemia
Hyperglycemia was evaluated in terms of its harshness and severity in relation to the maximum glucose level that the patients presented during the first 10 days after the start of treatment, or the first 10 days of hospitalization in patients without corticosteroids. Drug-associated hyperglycemia was not considered in patients admitted with uncontrolled levels of hyperglycemic greater than 200 mg/dL, as outlined in table 2, in concordance with the American Diabetes Association guideline (table 2).
Evaluation of corticosteroids associated with infections
The infections were evaluated taking into account the temporality, relating them to the administration of corticosteroids or hospital admission, in the control group. Documented infections during the first two days of treatment were not attributed to corticosteroids or hospitalization in the control group. It was confirmed that the infection identified in microbiological studies correlated with the therapeutic need for the administration of an antibiotic.
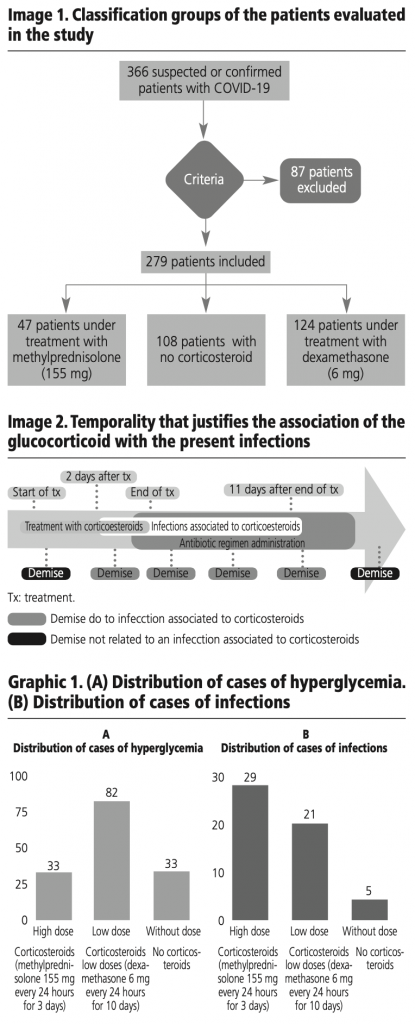
It was considered a harsh side effect if the patient died during the infectious process or during the administration of the antibiotic. A side effect was considered moderate when the patient only required taking an antibiotic and did not die, and severe when the patient required the administration of a double antibiotic regimen to treat the infection, or died during the infectious process as exemplified in image 2.
RESULTS
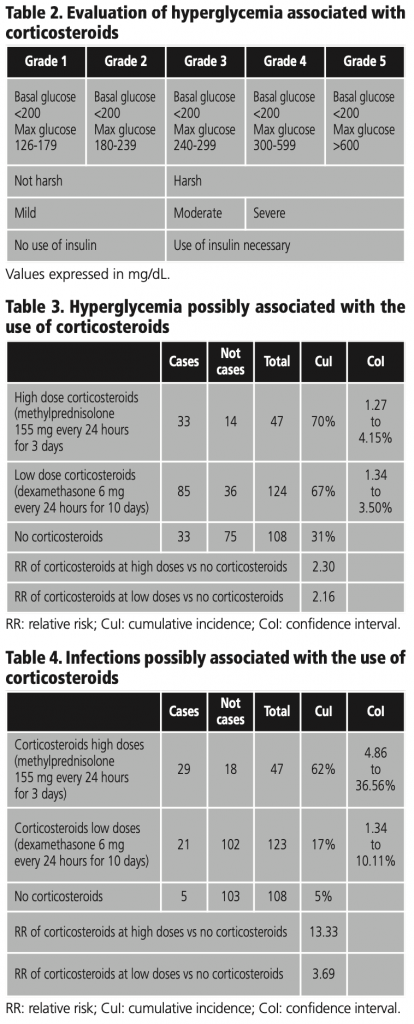
366 patients were evaluated, of whom 279 met the inclusion criteria. Of these, 47 patients were administered high doses of corticosteroids (methylprednisolone 155 mg average/day) for 3 days, 124 used low doses of corticosteroids (dexamethasone 6 mg/day) for 10 days and 108 did not use corticosteroids. The distribution of the cases with hyperglycemia was 33 in high doses and 82 with low doses of corticosteroids and both high and low doses have the similar distribution in cases of infections as exemplified in graphic 1.
We observed that the cumulative incidence of hyperglycemia in the group with high doses of corticosteroids (methylprednisolone 155 mg average/day) is 70%, low doses of corticosteroids (dexamethasone 6 m /day) is 67% and the control group is 31%, as represented in table 3.
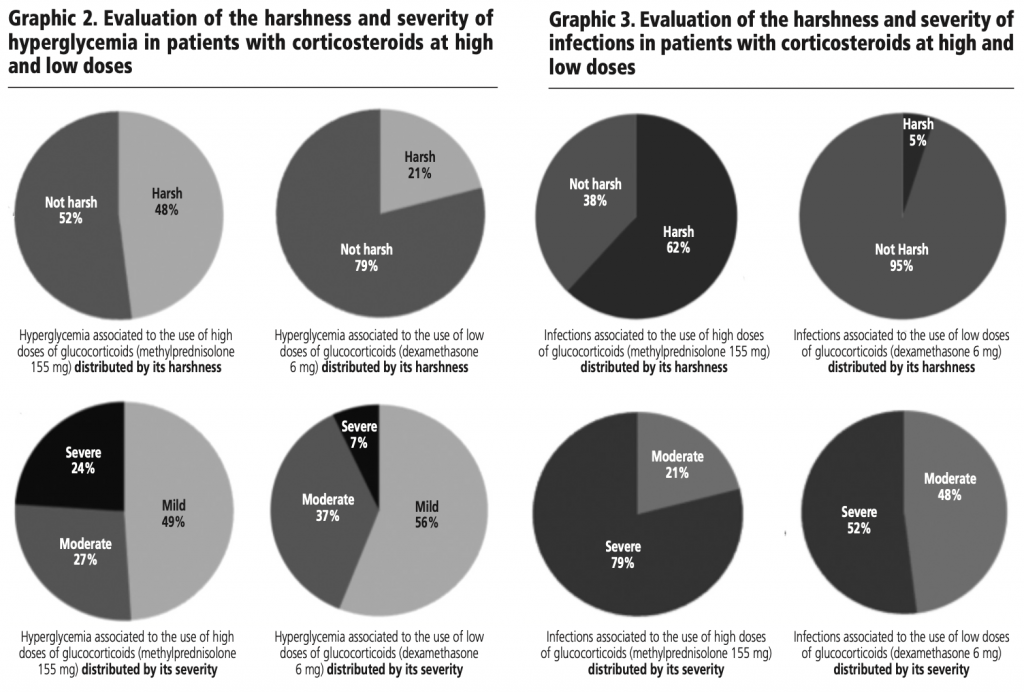
When evaluating the harshness and severity of hyperglycemia in the two groups with corticosteroids, it is observed that patients with high doses (methylprednisolone 155 mg average/day) present more harsh (48%) and more severe (24%) cases than those who were administered low doses (Dexamethasone 6 mg/day) as seen in graphic 2.
We observed that the cumulative incidence of infections in the group with high doses of corticosteroids (methylprednisolone 155 mg average/day) is 62%, low doses of corticosteroids (dexamethasone 6 mg/day) is 17% and the control group is 5%, as represented in table 4. The distribution of cases is exemplified in graphic 1 (B).
When evaluating the harshness and severity of infections in the two groups with corticosteroids, it is observed that patients with high doses (methylprednisolone 155 mg average/day) present more harsh (62%) and more severe (79%) cases than those who were administered low doses (dexamethasone 6 mg/day) as seen in graphic 3.
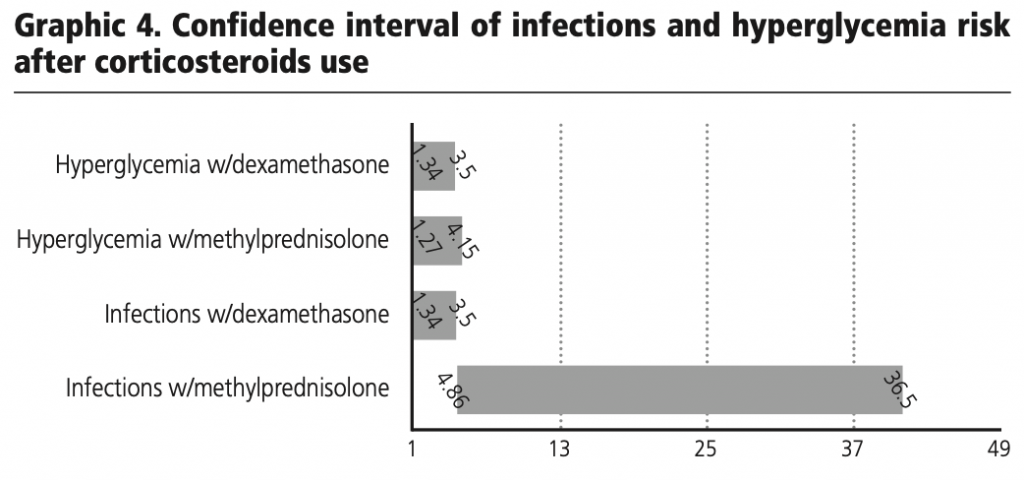
Based on the results or the 95% confidence interval calculation for estimating the risk of infections and hyperglycemia after the use of methylprednisolone and dexamethasone in the patients evaluated, it was observed that there is a risk of these effects occurring after the use of corticosteroids.
It is relevant to consider that this side effects could be conditioned by the presence of risk factors and previous comorbidities that we do not consider on the evaluation. Never the lees with the creatures we established on this study we can observed a grater harshness and severity in hyperglycemia and infections associated to high doses of corticosteroids (methylprednisolone 155 mg average/day) in COVID-19 patients.
CONCLUSIONS
Drugs are not completely safe, so the risk of side effects that represent harm or severity to the patient is always there. With this study, it is possible to establish a possible relationship between the use of high-dose corticosteroids and a greater distribution of harsh and severe hyperglycemia, as well as a greater number of infections with a greater harshness and severity, when compared with low-dose corticosteroids.
The use of glucocorticoids at high and low doses as a treatment for COVID-19 disease has been shown to be effective in reducing symptoms and days of hospitalization in these patients. However, based on the results obtained, it was concluded that the use of these drugs can cause changes in the basal glucose of patients, in addition to increasing the risk of contracting in-hospital infections due to immunosuppression secondary to the use of glucocorticoids. Despite the fact that the results of this study do demonstrate a relationship between the use of dexamethasone and methylprednisolone with the mentioned side effects, it is recommended to carry out a broader investigation and with a greater cutoff of the values used, in order to establish a closer relationship between the use of glucocorticoids with hyperglycemia and related infections.
Conflict of interests: The authors declare that they do not present a conflict of interest.
BIBLIOGRAPHY
1. Singhal, Tanu. (13 marzo 2020). A Review of Coronavirus Disease-2019 (COVID-19). The Indian Journal of Pediatrics. Vol 87 (4),281-286.
2. Solinas Cinzia, Perra Laura, Aiello Marco, et al. (24 junio 2020). A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine and growth factor reviews. Vol 54, 8-23.
3. Qing Ye, Bili Wang, Jianhua Mao. (24 marzo 2020). The pathogenesis and treatment of the Cytokine Storm in COVID-19. Journal of Infection. Vol 80, 607-613.
4. Naserghandi A, Allameh S, Saffarpour R. (13 abril 2020). All about COVID-19 in brief. New Microbes and New Infections, Vol 35 (C),100678.
5. Abdin Shifaa M, Elgendy Sara M, Alyammahi Shatha K, et al. (11 julio 2020). Tackling the cytokine storm in COVID-19, challenges and hopes. Vol. 257; 118054.
6. Escuela Andaluza de Salud Pública. (2020) Tratamientos para el COVID-19: sospechas de reacciones adversas.
7. Agencia Española de Medicamentos y Productos Sanitarios. (23 octubre 2020). Sospechas de reacciones adversas notificadas con tratamientos utilizados en COVID-19.
8. RECOVERY dexamethasone. Randomised Evaluation of COVID-19 Therapy.
9. Centro de Información de Medicamentos. Ficha técnica: Dexametasona. Agencia Española de Medicamentos y Productos Sanitarios.
10. Asociación española de pediatría: Comité de medicamentos. Pediamécum AEP: Dexametasona. Mayo 2016.
11. Morieri Mario L, Fadini Gian P, Boscari Federico, et al. Hyperglycemia, glucocorticoid therapy and outcome of COVID-19. Diabetes research and clinical practice. (2020) 108449.
12. Zabuliene Lina. (25 marzo 2020). Hyperglycemia and the novel Covid-19 infection: Possible pathophysiologic mechanisms. Vol 139, 109699.
13. Balanciano, Giselle. Carrasco, Gabriela. García, Darío, et al. (agosto 2020). Uso de dexametasona en pacientes internados con COVID-19. Informe de evaluación de tecnología sanitaria. Red Argentina Pública de Evaluación de Tecnologías Sanitarias.
14. Cain Derek W, Cidlowski John A. (Julio 2020). After 62 years of regulating immunity, dexamethasone meets COVID-19. Nature reviews: Immunology.
15. García Milián, Ana Julia. (30 junio 2016). Farmacovigilancia hospitalaria. Revista Cubana de Oftalmología. Vol. 29 (4), 1561-3070.
16. Romagnoli Stefano, Peris Adriano, De Gaudio Raffaele, et al. (1 octubre 2020). SARS-CoV-2 and COVID-19: From de Bench to he Beside. Physiological Reviews. Vol 100 (4), 1455-1466.
17. Twomey Julianne D, Luo Shen, Dean Alexis Q, et al. (24 octubre 2020). Covid-19 update: The race to therapeutic development. Drug Resistance Updates. Vol. 53, 100733.
18. Asselah Tarik, Durantel David, Pasmant Eric, et al. (8 octubre 2020). COVID-19: Discovery, diagnosis and drug development. Journal of Hepatology 2021. Vol. 74, 16-184.
19. Cascella Marco, Rajnik Michael, Cuomo Arturo, et al. (4 octubre 2020). Features, Evaluation, and Treatment of Coronavirus. StatPearls Publishing.
20. Herrera-Lasso Regás Valeria, Dordal Culla María T, Lleonart Bellfill Ramón. (9 julio 2020). Reacciones adversas a fármacos utilizados en el tratamiento específico de la infección por SARS-CoV-2. Med Clin 2020. Vol. 155(10):448-453.
21 Organización mundial de la salud. (2 septiembre 2020). Corticoides para el tratamiento de la COVID-19. Organización Mundial de la Salud 2020.
22 Aikaterini Papamanoli, Yoo Jeanwoo, Prabhjot Grewal. (Septiembre 2020). High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. European Society for Clinical Investigation Journal Foundation 2020.
23 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7837645/pdf/cureus-0012-00000012330.pdf.
____