Martínez-Sesmero JM1, Crespo-Diz C2, Cerezales M3, Crespo C4,5, Guigini MA6, Schoenenberger-Arnaiz JA7,8
- Chief of Hospital Pharmacy Department. Hospital Clínico San Carlos. Madrid.
- Chief of Hospital Pharmacy Department. Área Sanitaria Pontevedra e O Salnés / Complexo Hospitalario Universitario de Pontevedra, Pontevedra
- Axentiva Solutions S.L., Oviedo
- Axentiva Solutions S.L., Barcelona
- G.M. Statistics Department. Universidad de Barcelona, Barcelona
- Fresenius Kabi España, S.A.U, Barcelona
- Chief of pharmacy department. Regió Sanitària de Lleida, Lleida
- Associated pharmacology profesor. Universitat de Lleida, Lleida
Fecha de recepción: 11/08/2023 – Fecha de aceptación: 06/09/2023
Correspondencia: Dr. Carlos Crespo Diz · Xefe de Servizo de Farmacia · Área Sanitaria Pontevedra e O Salnés · 36164 – Pontevedra · Email:Carlos.Crespo.Diz@sergas.es
____
Introduction: Psoriasis (Ps), rheumatoid arthritis (RA), Crohn’s disease (CD), and ulcerative colitis (UC) are the most prevalent immune-mediated inflammatory diseases (IMIDs) in Spain. Biological treatments have contributed to improve their outcomes but until the arrival of biosimilars, such as adalimumab (ADA), biologics’ high cost was a barrier to their prescription. Our objective was to assess the cost-effectiveness of ADA and clinical alternatives for IMIDs.
Methods: A cost-effectiveness model was built based on systematic review of network meta-analysis (NMA) from a hospital perspective with a 1-year time horizon. The systematic review of NMA was performed (2015-2021) in English and Spanish following Cochrane guidelines. Two reviewers evaluated the inclusion of the studies and assessed their quality using the PRISMA-NMA Checklist. Costs (€2021) were obtained from Spanish drug cost datasets and literature. Effectiveness was measured as the number-needed-to-treat (NNT) versus placebo (PLC). Efficiency was cost per response vs. PLC. A cost-effectiveness analysis between ADA and the suitable alternatives was performed with additional deterministic and probabilistic sensitivity analyses.
Results: Six meta-analyses were included fulfilling 80% of the PRISMA-NMA Checklist items. For Ps, ADA was the most cost-effective, it had the lowest cost/NNT in all PASI (between €8.338,89 and €19.944,89). For RA (ACR-20/50), CD and UC, there were no statistically significant effectiveness differences, and ADA, the cheapest treatment (between €4.529,20 and €5.230,99), was considered the most cost-effective. Tocilizumab (€4.275,46) showed a lower cost/DAS28 reduction in RA.
Conclusions: ADA was the most cost-effective option in Ps, RA (ACR 20/50), CD and UC. For RA (DAS28), tocilizumab was more cost-effective.
Keywords: Adalimumab, immune-mediated inflammatory diseases, biologics, cost-effectiveness
Análisis coste-efectividad de adalimumab en pacientes con enfermedades inflamatorias inmunomediadas en España
Introducción: La psoriasis (Ps), artritis reumatoide (AR), enfermedad de Crohn (EC) y colitis ulcerosa (CU) son las enfermedades inflamatorias inmunomediadas (IMIDs) más prevalentes. Los tratamientos biológicos han contribuido a mejorarlas, pero su elevado coste era una barrera para su prescripción hasta la llegada de los biosimilares, como adalimumab (ADA). Nuestro objetivo fue evaluar el coste-efectividad de ADA y las alternativas terapéuticas para IMIDs en España.
Métodos: Se definieron las alternativas terapéuticas y las medidas de efectividad mediante un panel de expertos. La efectividad de los tratamientos se obtuvo de la literatura, dos revisores evaluaron los estudios y valoraron su calidad (PRISMA-NMA). Se construyó un modelo coste-efectividad basado en metaanálisis desde una perspectiva hospitalaria con un horizonte temporal de 1 año. Los costes (2021€) se obtuvieron de los conjuntos de datos de costes de medicamentos españoles y de la literatura. La efectividad se midió como número necesario a tratar (NNT) frente a placebo (PLC) y la eficacia como coste por respuesta frente a PLC. Se realizó un análisis de coste-efectividad y un análisis de sensibilidad determinístico y probabilístico.
Resultados: Seis metaanálisis cumplieron el 80% de los ítems PRISMA-NMA. Para Ps, ADA fue la opción más coste-efectiva, con el menor coste/NNT (8.338,89€-19.944,89€). Para AR, EC y CU, no hubo diferencias de eficacia estadísticamente significativas, y ADA, al ser el tratamiento más barato (4.529,20€-5.230,99€), fue la opción más coste-efectiva. Tocilizumab mostró un menor coste por reducción de DAS28 en AR (4.275,46€).
Conclusiones: ADA fue la opción más coste-efectiva para Ps, AR, EC y CU. Para AR (DAS28), tocilizumab fue más eficiente.
Palabras clave: Adalimumab, enfermedades inflamatorias inmunomediadas, biológicos, coste-efectividad
____
Introduction
Immune-mediated inflammatory diseases (IMIDs) and their clinical manifestations represent a humanistic burden1 as well as a high economic burden on healthcare systems worldwide, this is due to the high resource consumption in the appropriate management of affected patients2,3. The prevalence of these diseases and multimorbidity are increasing exponentially4, and until now, healthcare systems have focused mainly on the treatment of acute episodes, with chronic management being relegated to a secondary role5.
IMIDs are chronic illnesses that generally begin to debut when the patient is between 20 and 50 years old, an age when patients have an active working life1. In Spain, a recent epidemiological study6 showed that IMIDs joint prevalence is about 6,39% (3 million people). Among the different pathologies under the IMIDs umbrella, the most important were Psoriasis (Ps), Rheumatoid Arthritis (RA), and gastrointestinal IMIDs (Crohn’s disease (CD) and ulcerative colitis (UC)). In fact, Ps prevalence ranges from 1,8%7 to 2,69%6, and accounts for a notoriously high resource consumption rate partially due to a greater number of comorbidities. In the case of RA, the prevalence is reduced to 1,07%6, although its debilitating effect in patients represents a high humanistic and economic burden8. Finally, CD and UC reached a joint prevalence
of 0,78%.
The development of biological drugs 20 years ago changed the management of IMIDs in these patients. Their lives were improved substantially as the disease is better controlled, less corticoids are consumed9, and a better overall quality of life is achieved10. Used at early onset, these treatments can reduce the impact of the disease11. In addition to biologic therapy, biosimilars have provided further optimization by maintaining the benefit-risk profile and reducing drug costs3,12.
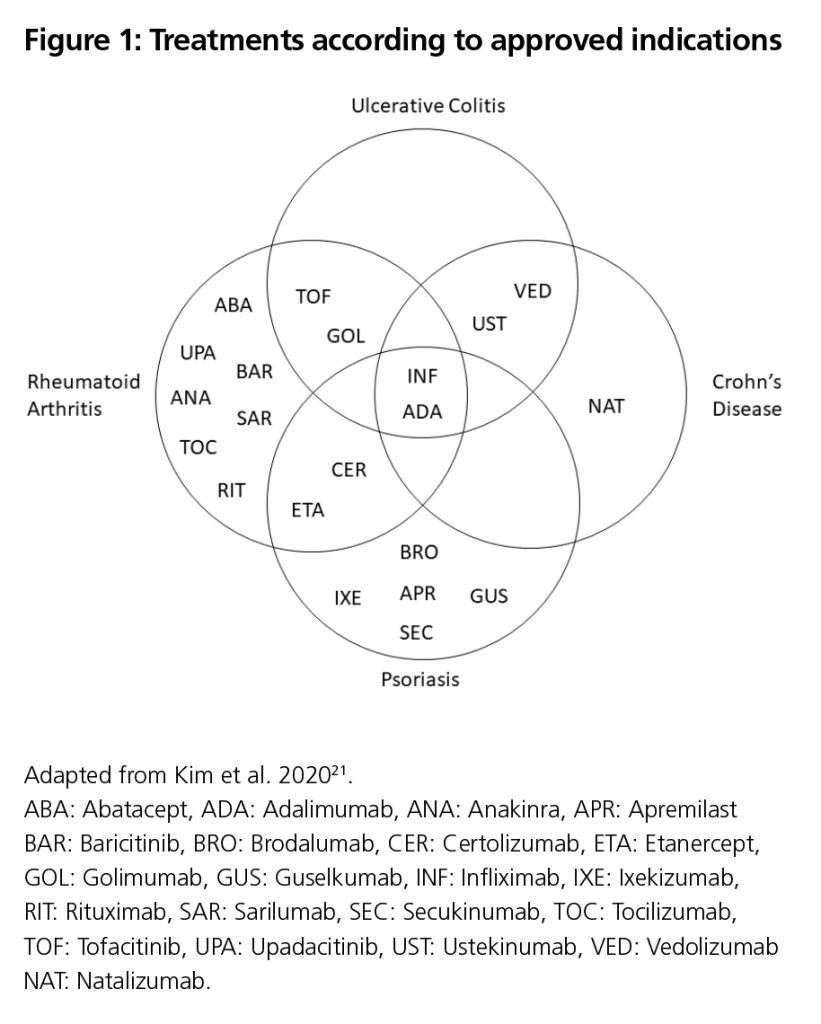
Currently, there are numerous biological and biosimilar therapeutic alternatives for the management of these diseases. The most common options for the treatment of IMIDs in Spain according to their approved indications are: abatacept, adalimumab, anakinra, apremilast baricitinib, brodalumab, certolizumab, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, sarilumab, secukinumab, tocilizumab, tofacitinib, upadacitinib, ustekinumab, and vedolizumab9,13. Among these alternatives, adalimumab presents a very versatile profile, being an approved therapeutic alternative for these IMIDs (Figure 1).
Due to the considerable variety of treatment options available, to make a well- informed decision for both the patient and the healthcare system, it is crucial to generate evidence that can support this decision-making process.
Therefore, the aim of this study was to estimate the cost-effectiveness of adalimumab in Ps, RA, CD and UC compared to the most common treatments in Spain from the Hospital Pharmacy perspective.
Methods
A cost-effectiveness model was constructed comparing adalimumab vs current clinical alternatives for each pathology. Studies with patients using biological drugs for Ps, RA, CD or UC were included in the model.
Literature Review
A systematic literature review was conducted (Supplemental Table 1) following Cochrane guidelines in key databases: MEDLINE, Embase, Database of Abstracts of Reviews of Effects, National Health Service Economic Evaluation Database, and Health Technology Assessment. The search was performed following the PICO-S-T approach as follows.
- P (Patient): Patients suffering: Ps, RA, CD, and UC.
- I (Intervention): adalimumab.
- C (Comparator): Only active substances reimbursed and currently used in clinical practice in Spain for each of the indications were included: baricitinib, certolizumab, etanercept, golimumab, guselkumab, infliximab, ixekizumab, secukinumab, tocilizumab, tofacitinib, upadacitinib, ustekinumab, vedolizumab
- O (Outcome): Effectiveness indicators: Psoriasis Area and Severity Index (PASI) for Ps; Disease Activity Index (DAS28) and American College of Rheumatology (ACR) for RA; Crohn Disease Activity Index (CDAI), clinical remission and clinical response for CD; and Mayo scale (clinical remission and clinical response) and haemorrhage for UC.
- S (Study Type): Terms involving direct or indirect meta-analyses: Indirect Treatment Comparison, Network Meta-Analysis, Mixed-Treatment Comparison.
- T (Time Frame): From 2015 to September 2021.
Searches were limited to articles published in English and Spanish. Attempts were made to identify the full texts of all conference abstracts, however, where none were available, abstracts were excluded due to insufficient information being reported. In addition, a hand search of the identified literature references was conducted. All references were downloaded, and duplicates were removed.
The review of titles and abstracts was performed blindly by two investigators experienced in systematic reviews. In the case of disagreement over a reference, a consensus was reached with the participation of a third investigator. The quality of those references that met the inclusion criteria was assessed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension for network meta-analysis (NMA)
guidelines14.
For the studies finally selected, information was obtained on the comparators, the effectiveness indicator, the pathology, and the meta-analysis method used.
Costs
In order to estimate the pharmacological cost of the treatments, the “BOT PLUS” database from the general pharmaceutical council of Spain was used at ex-factory price15. From this price, the discount from Royal Decree 08/201016 was applied and the price for the annual treatment was estimated using the appropriate dosage for each treatment.
Since drugs may be dual-priced or involved in public tenders, a panel of representative hospital pharmacists in Spain was constructed to estimate the range of potential discounts for each of the treatments evaluated. Based on the consultation with the panel of pharmacists, the minimum, average, and maximum discount for each treatment applicable to the ex-factory price was estimated and subsequently validated at a consensus meeting. In this way, an attempt was made to estimate what the real pharmacological cost could be at hospital level.
For the estimation of non-pharmacological costs, information associated with each pathology was obtained from the literature. In this regard, for Ps the study by Alfageme et al. 201617 was used, for RA the study by León et al. 201618, and for UC the study by Trigo-Vicente et al. 202019 . For CD, no cost or cost-effectiveness study of sufficient quality was found. Therefore, the cost of CD was assumed to be similar to the cost of UC. All costs were updated to the 2020 consumer price index from the national statistics institute20.
Total costs were calculated from the sum of the two previous costs:
Cost-effectiveness analysis
The measures of effectiveness analysed were the Number Needed to Treat (NNT) versus Placebo (PLC) directly or calculated from the response probabilities (Relative Risk or Odds Ratio). The results of the cost-effectiveness analysis were expressed using the incremental cost-effectiveness ratio (ICER) of adalimumab vs alternatives, calculated using the following formula (Supplemental Figure 1):
An alternative was defined as the most cost-effective when it was less costly and more effective (dominant) than the rest of the evaluated alternatives. Alternatively, when there was no dominant option:
- if statistically significant differences in effectiveness were seen, a cost-effectivity analysis was performed. The alternative with the lowest cost per response against PLB was defined as the most cost-effective or,
- if there was no evidence of statistically significant differences in effectiveness, a cost-minimization analysis was performed. An alternative was defined as the most cost-effective when it was identified as the alternative with the lower cost.
Sensitivity analysis
A deterministic sensitivity analysis of the response variables was carried out based on the range of discounts that could be assumed for the drugs in each indication. In addition, a probabilistic sensitivity analysis was performed for each pathology to estimate the probability that adalimumab was the most cost-effective treatment, modifying the parameters according to their plausible range. The distributions for costs (gamma) and probabilities (beta) were assumed as indicated in the main guidelines21.
Results
Screening
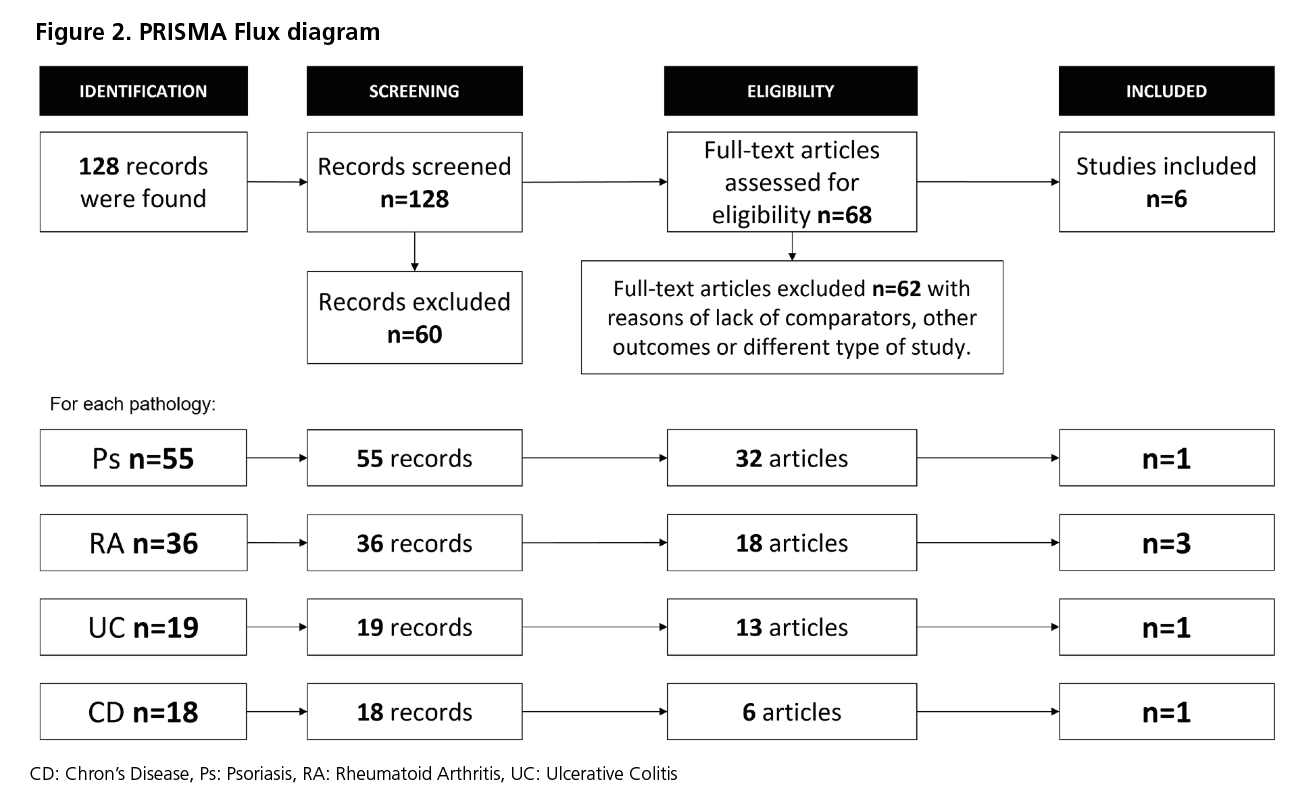
The initial search led to 128 studies, mainly from Ps and RA (Supplemental Table 2). After peer review, 47% of them were discarded as clinical trials, observational studies, or opinion articles. When reviewing the 68 eligible articles in detail, 62 were discarded for not including adalimumab or the main target treatments, for using a different clinical outcome, and for conducting analyses other than direct or indirect meta-analyses. No studies were available for some drugs (Brodalumab, and Sarilumab). (Figure 2)
Finally, the meta-analyses included were Armstrong22 for PS, Leil23, Song34 and Tarp35 for RA, Singh26 for CD, and Lohan27 for UC (Table 1).
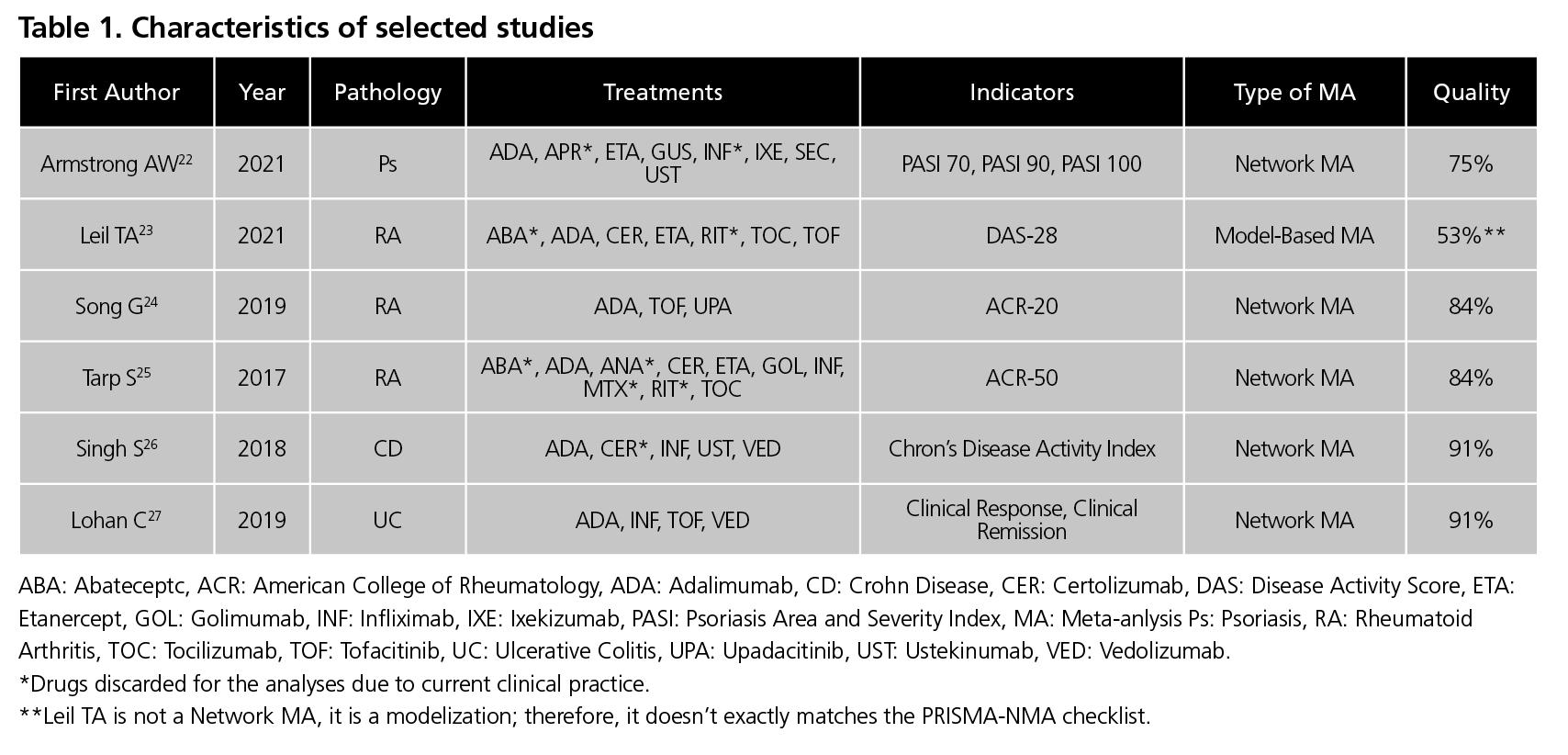
Effectiveness
When evaluating PASI75, PASI90 and PASI100 for Ps, guselkumab, ixekizumab, and secukinumab were found to have a statistically superior response rate to adalimumab, etanercept, and ustekinumab22.
For RA, it was noted that each included study had a different measure of effectiveness (DAS-28 Leil23, ACR20 Song24 and ACR50 Tarp25). Focusing on the treatments, tocilizumab showed a significantly higher reduction in DAS-28 than adalimumab, certolizumab, etanercept, and tofacitinib25. However, for the ACR20 and ACR50 effectiveness indicator, no statistically significant differences were observed between adalimumab, certolizumab, etanercept, golimumab, infliximab, tocilizumab, tofacitinib, and upadacitinib 23,24.
For CD, no significant differences were detected between adalimumab, infliximab, ustekinumab, and vedolizumab26.Similarly, for UC, no significant differences in Mayo scale (clinical remission and clinical response) were observed for adalimumab, infliximab, tofacitinib and
vedolizumab27.
Cost-effectiveness
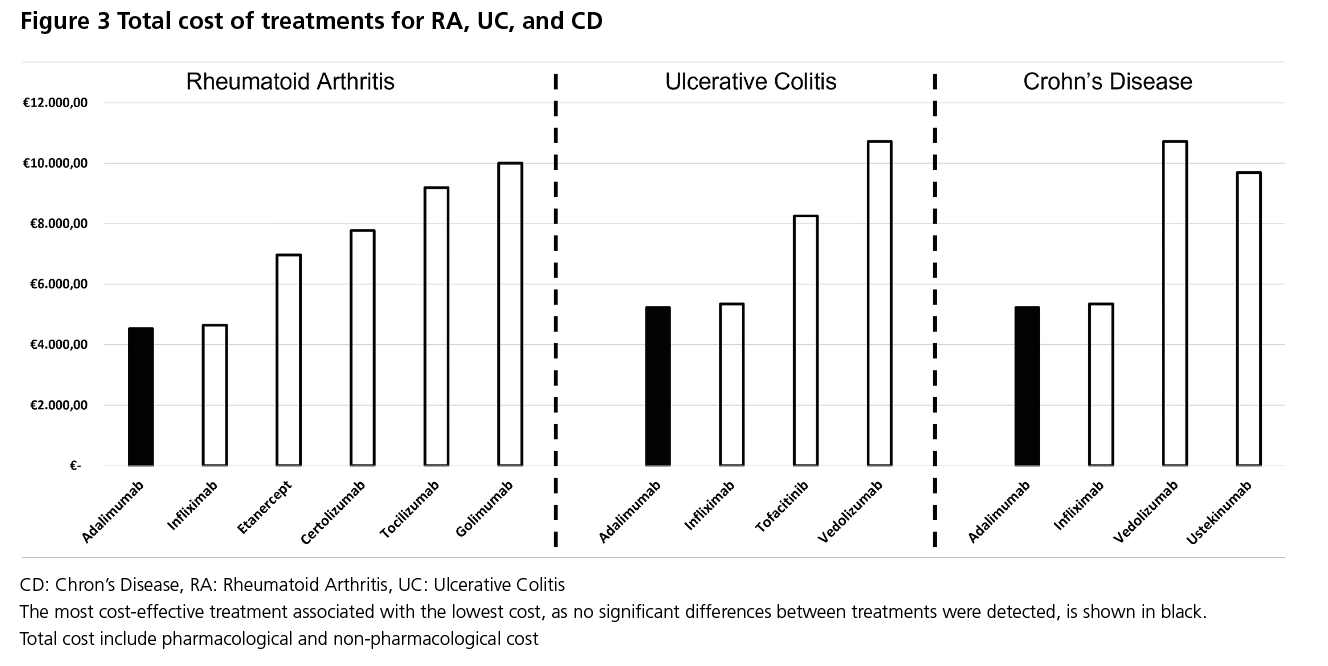
For Ps, adalimumab showed a cost per NNT vs PLC in PASI75 of €8,338.89, lower than the three treatments with a statistically higher effectiveness: secukinumab (€14,626.17), ixekizumab (€12,285.85), and guselkumab (€13,236.66). Therefore, ADA was the most cost-effective option. In PASI90 and 100, the results followed the same trend, but with less difference between ADA (PASI90, €10,584.62; PASI100 €19,944.89) and the rest of treatments, although ADA was always the most cost-effective option.
When evaluating the total annual cost of RA treatments, adalimumab was found to have a cost of €4,529 compared to €4,650-€10,001 for other TNF-alpha/Interleukin inhibitors, therefore making it the treatment with the lowest cost compared to the alternatives (Figure 3). If ACR20 or ACR50 are considered as the measure of effectiveness, as no differences in efficacy were observed, a cost-minimisation analysis was performed, making it the most cost-effective treatment. On the other hand, if the measure of effectiveness is DAS-28, then the cost per reduction of DAS-28 for tocilizumab is €3,763 compared
to adalimumab
In CD, adalimumab reduced the total direct annual cost by €120.80 compared to infliximab, by €4,463.24 compared to ustekinumab, and by €5,483.48 compared to vedolizumab (Figure 3). In terms of effectiveness, no significant differences were detected between treatments, consequently, in a cost-minimisation analysis, adalimumab was the most cost-effective treatment.
Finally, in the case of UC, adalimumab reduced the total direct annual cost by €120.80 versus infliximab, €3,028.16 versus tofacitinib, and €5,483.48 versus vedolizumab (Figure 3). Given that no differences in efficacy were observed, a cost-minimisation analysis was performed between the treatments, which showed adalimumab to be the most cost-effective treatment.
Sensitivity Analysis
Modifying the discount range between the minimum and maximum showed the same trend observed in the overall results, except when extreme discounts were applied for infliximab, therefore making it the most cost-effective treatment. However, multivariate Monte Carlo analysis showed that adalimumab was the treatment most likely to be cost-effective over the others, 3-4 times more likely than infliximab.
Discussion
Biological drugs remain an essential pillar in the management of IMIDs, however, until the introduction of biosimilars they represented a significant pharmacological cost. Due to their economic impact, their use has been limited in some cases28. With the increasing use of biosimilars, the need to assess the efficiency of the therapeutic arsenal is reopened, as there have been some pharmacoeconomic analyses with negative results for biologic drugs in the past29, and its early initiation in patients has yet to be evaluated in cost-effectiveness studies30.
With the development of biosimilars, competition has increased in the IMIDs market, therefore reducing the costs of biological treatments without reducing their effectiveness. Moreover, it is worth considering that improving the efficiency of the healthcare system allows resources to be invested in more expensive drugs in the neediest populations.
Currently there is inaccurate information on drug prices at the hospital level due to the availability of dual pricing thus reducing the accuracy of cost-effectiveness studies. The study by Espín et al. reports that for Spain, the differences between net hospital expenditure and aggregate expenditure range between 22% and 34%, without considering other mandatory discounting. This value is similar to the 18% observed in the same study for the EU5 (France, Germany, Italy, Spain, and the United Kingdom)31.
In our study, a panel of experts was conducted in order to identify the potential discount range for each drug. Doing so, our study tries to provide an accurate description of the cost-effectiveness of the drugs evaluated. Nevertheless, we should consider that hospital price negotiation depends on many factors such as the type of hospital, the number of patients, or its relationship with the pharmaceutical industry, hence variations may appear between centres and in time.
In terms of cost-effectiveness studies, our results, although with a limited time horizon, show a similar trend to the study by Trigo-Vicente et al 202019 for UC. In that study, where a Markov model with a 10-year time horizon and similar treatments was performed, adalimumab was the most cost-effective treatment in patients with moderate-severe UC in Spain.
Conclusion
According to the pharmacoeconomic models developed for Ps, RA, CD, and UC, adalimumab is the most cost-effective treatment compared to the alternatives in IMIDs, except when the DAS-28 marker is used in RA, where the additional cost of tocilizumab is offset by its greater effectiveness.
Conflicts of Interest: JM Martínez-Sesmero has earned fees from: Abbvie, Pfizer, Fresenius Kabi, Galapagos, Lilly, and Novartis. JA Schoenenberger-Arnaiz has earned fees from: Biogen, AstraZeneca, and LEO Pharma. C Crespo-Diz has earned fees from: Abbvie, Almirall, Amgen, AstraZeneca, Bayer, Biogen, BMS, Fresenius Kabi, Gilead, GSK, Grifols, Janssen-Cilag, Kern Pharma, GSK, Novartis, Novo Nordisk, Pfizer, Roche, Shire, SOBI, Takeda, and UCB. M Cerezales and C Crespo work in Axentiva Solutions, a consultancy firm working for several pharmaceutical companies. M.A Guigini works for Fresenius Kabi España, S.A.U.
References
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020 Oct 17;396(10258):1204–22.
- Burisch J, Vardi H, Schwartz D, Friger M, Kiudelis G, Kupčinskas J, et al. Health-care costs of inflammatory bowel disease in a pan-European, community-based, inception cohort during 5 years of follow-up: a population-based study. Lancet Gastroenterol Hepatol. 2020 May 1;5(5):454–64.
- Brunet E, Vela E, Melcarne L, Clèries M, Pontes C, Llovet LP, et al. Time Trends of Crohn’s Disease in Catalonia from 2011 to 2017. Increasing Use of Biologics Correlates with a Reduced Need for Surgery. J Clin Med. 2020 Sep 8;9(9):2896.
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 1;380(9859):2197–223.
- Marín-Jiménez I, Casellas F, Cortés X, García-Sepulcre MF, Juliá B, Cea-Calvo L, et al. The experience of inflammatory bowel disease patients with healthcare. Medicine (Baltimore). 2019 Apr 1;98(14):e15044.
- Puig L, Ruiz de Morales JG, Dauden E, Andreu JL, Cervera R, Adán A, et al. [Prevalence of ten Immune-mediated inflammatory diseases (IMID) in Spain]. Rev Esp Salud Publica. 2019 Mar;93.
- Puig L, Ferrándiz C, Pujol RM, Vela E, Albertí-Casas C, Comellas M, et al. Carga de la psoriasis en Cataluña: datos epidemiológicos, comorbilidades asociadas, uso de recursos sanitarios e incapacidad laboral. Actas Dermosifiliogr. 2021 May 1;112(5):425–33.
- Andrade P, A Sacristan J, Luz Rentero M, Hammen V, Dilla T. The Burden of Rheumatoid Arthritis in Spain. Heal Econ Outcome Res Open Access. 2017;03(01).
- McInnes IB, Gravallese E. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21:680–6.
- Spierings J, Sloeserwij A, Vianen ME, de Boer JH, Sigurdsson V, van de Wijgert JHHM, et al. Health-related quality of life in patients with immune mediated inflammatory diseases: A cross-sectional, multidisciplinary study. Clin Immunol. 2020 May;214:108392.
- Baumgart DC, Misery L, Naeyaert S, Taylor PC. Biological Therapies in Immune-Mediated Inflammatory Diseases: Can Biosimilars Reduce Access Inequities? . Vol. 10, Frontiers in Pharmacology . 2019. p. 279.
- Calleja-Hernández MÁ, Martínez-Sesmero JM, Santiago-Josefat B. Biosimilars of monoclonal antibodies in inflammatory diseases and cancer: Current situation, challenges, and opportunities. Farm Hosp. 2020 Jun 1;44(3):100–8.
- Kuek A, Hazleman BL, Ostor AJK. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007 Apr;83(978):251–60.
- Hutton B, Salanti G, Caldwell D, Chaimani A, Schmid C, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015 Jun 2;162(11):777–84.
- BOTPLUS [Internet]. [cited 2023 Aug 8]. Available from: https://botplusweb.farmaceuticos.com/
- Boletín Oficial del Estado. Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. 2010. p. 126:45070.
- Alfageme Roldán F, Bermejo Hernando A, Calvo González JL, Marqués Sánchez P. [Cost Effectiveness of Treatments of Psoriasis with a PASI 75 and one Period of 12 Weeks]. Rev Esp Salud Publica. 2016 Apr;90:E15.
- Leon L, Abasolo L, Fernandez-Gutierrez B, Jover JA, Hernandez-Garcia C. Costes médicos directos y sus predictores en la cohorte “Variabilidad en el manejo de la artritis reumatoide y las espondiloartritis en España.” Reumatol Clínica. 2018 Jan;14(1):4–8.
- Trigo-Vicente C, Gimeno-Ballester V, López-Del Val A. Cost-effectiveness analysis of infliximab, adalimumab, golimumab, vedolizumab and tofacitinib for moderate to severe ulcerative colitis in Spain. Eur J Hosp Pharm. 2020 Nov;27(6):355–60.
- Instituto Nacional de Estadística. Consumer Price Index: National results. 2020.
- Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force – PubMed. 2020.
- Armstrong AW, Soliman AM, Betts KA, Wang Y, Gao Y, Puig L, et al. Comparative Efficacy and Relative Ranking of Biologics and Oral Therapies for Moderate-to-Severe Plaque Psoriasis: A Network Meta-analysis. Dermatol Ther (Heidelb). 2021 Jun;11(3):885–905.
- Leil TA, Lu Y, Bouillon‐Pichault M, Wong R, Nowak M. Model‐Based Meta‐Analysis Compares DAS28 Rheumatoid Arthritis Treatment Effects and Suggests an Expedited Trial Design for Early Clinical Development. Clin Pharmacol Ther. 2021 Feb;109(2):517–27.
- Song GG, Choi SJ, Lee YH. Comparison of the efficacy and safety of tofacitinib and upadacitinib in patients with active rheumatoid arthritis: A Bayesian network meta-analysis of randomized controlled trials. Int J Rheum Dis. 2019;22(8):1563–71.
- Tarp S, Furst DE, Dossing A, Østergaard M, Lorenzen T, Hansen MS, et al. Defining the optimal biological monotherapy in rheumatoid arthritis: A systematic review and meta-analysis of randomised trials. Semin Arthritis Rheum. 2017 Jun;46(6):699–708.
- Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther. 2018 Aug;48(4):394–409.
- Lohan C, Diamantopoulos A, LeReun C, Wright E, Bohm N, Sawyer LM. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019 Jul;6(1):e000302.
- Kim H, Alten R, Avedano L, Dignass A, Gomollón F, Greveson K, et al. The Future of Biosimilars: Maximizing Benefits Across Immune-Mediated Inflammatory Diseases. Drugs. 2020 Feb;80(2):99–113.
- Joensuu JT, Huoponen S, Aaltonen KJ, Konttinen YT, Nordström D, Blom M. The Cost-Effectiveness of Biologics for the Treatment of Rheumatoid Arthritis: A Systematic Review. Coles JA, editor. PLoS One. 2015 Mar;10(3):e0119683.
- Jansen JP, Incerti D, Mutebi A, Peneva D, MacEwan JP, Stolshek B, et al. Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J Med Econ. 2017 Jul;20(7):703–14.
- Espin J, Schlander M, Godman B, Anderson P, Mestre-Ferrandiz J, Borget I, et al. Projecting Pharmaceutical Expenditure in EU5 to 2021: Adjusting for the Impact of Discounts and Rebates. Appl Health Econ Health Policy. 2018 Dec;16(6):803–17.
____