Hilario Martínez-Barros1, Marina Sánchez-Cuervo1, Noelia Martínez Jáñez2, Ana Sendra García3, Ana María Álvarez-Díaz1
1Department of Pharmacy, Hospital Universitario Ramón y Cajal, Madrid, Spain
2Department of Medical Oncology, Hospital Universitario Ramón y Cajal, Madrid, Spain
3Department of Pharmacy, Hospital Universitario Doctor Peset, Pharmacy, Valencia, Spain
Fecha de recepción: 25/09/2022 – Fecha de aceptación: 25/10/2022
Correspondence: Hilario Martínez-Barros, Pharmacy, Hospital Universitario Ramón y Cajal. hilario.martinez@salud.madrid.org.
____
ABSTRACT
OBJECTIVE: Palbociclib was approved, combined with hormone therapy, as first-line treatment of hormone receptor-positive, HER2-negative locally advanced or metastatic breast cancer (MBC) based on the results of the PALOMA-2 clinical trial. The objective of this study is to determine its effectiveness, safety, and adherence in real clinical practice in this indication in our institution.
METHODS: We conducted a retrospective, longitudinal, single-centre study that included all patients who started palbociclib as first-line treatment for locally advanced or MBC between January 2018 and August 2019. Progression-free survival (PFS) was the primary endpoint of effectiveness. Adverse events (AE) and their grade, treatment modifications, and permanent discontinuations due to AE were the primary safety endpoints. Adherence was measured according to the proportion of days covered (PDC).
RESULTS: With a median follow-up of 21.7 months for the primary endpoint of effectiveness, a PFS of 27.4 months (95%CI: 15.5-35.5) was obtained compared to 27.6 (95%CI: 22.4-30.3) in the experimental cohort of PALOMA-2. Regarding its safety profile, despite a similar proportion of grade 3-4 AE (82.3% vs 79.3%) and permanent discontinuations due to AEs (13.7% vs 12.2%), cycle delays (84.4% vs 70.9%) and dose reductions (64.7% vs 39.4%) were higher compared to PALOMA-2. Medication PDC was 99.4%.
CONCLUSIONS: These results suggest that the effectiveness and safety of palbociclib as first-line treatment of locally advanced or MBC is similar to the reported in PALOMA-2, despite differences in the number of treatment modifications.
Keywords: Breast neoplasms, Medical Oncology, Antineoplastic agents, Drug-related side effects and adverse reactions, Safety
Eficacia y seguridad de Palbociclib como tratamiento de primera línea en el cáncer de mama avanzado
RESUMEN
OBJETIVO: Palbociclib fue aprobado, en combinación con terapia hormonal, como tratamiento de primera línea del cáncer de mama metastásico (CMM) o localmente avanzado con receptores hormonales positivos y HER2 negativo, en base a los resultados del ensayo clínico PALOMA-2. El objetivo de este estudio es determinar su eficacia, seguridad y adherencia en la práctica clínica real en esta indicación en nuestro centro.
MÉTODOS: Realizamos un estudio retrospectivo, longitudinal y unicéntrico que incluyó a todas las pacientes que iniciaron palbociclib como tratamiento de primera línea para el CMM o localmente avanzado entre enero de 2018 y agosto de 2019. La supervivencia libre de progresión (SLP) fue la variable principal de la efectividad. Los efectoss adversos (EA) y su grado, las modificaciones del tratamiento y las interrupciones permanentes debidas a EA fueron las variables principales de seguridad. La adherencia se midió según la proporción de días cubiertos (PDC).
RESULTADOS: Con una mediana de seguimiento de 21,7 meses para la variable principal de efectividad, se obtuvo una SLP de 27,4 meses (IC 95%: 15,5-35,5) frente a 27,6 (IC 95%: 22,4-30,3) en la cohorte experimental de PALOMA-2. En cuanto a su perfil de seguridad, a pesar de una proporción similar de EA de grado 3-4 (82,3% frente a 79,3%) y de interrupciones permanentes debidas a EA (13,7% frente a 12,2%), los retrasos de inicio de los ciclos (84,4% frente a 70,9%) y las reducciones de dosis (64,7% frente a 39,4%) fueron mayores en comparación con PALOMA-2. La PDC de la fue del 99,4%.
CONCLUSIONES: Estos resultados sugieren que la efectividad y la seguridad de palbociclib como tratamiento de primera línea del CMM o localmente avanzado es similar a la obtenida en PALOMA-2, a pesar de las diferencias en el número de modificaciones del tratamiento.
PALABRAS CLAVE: Neoplasias de mama, Oncología médica, Agentes antineoplásicos, Efectos secundarios y reacciones adversas relacionados con los medicamentos, Seguridad
____
INTRODUCTION
Breast cancer (BC) was the most diagnosed cancer in the world in 2020, and it is estimated that it will be the second most diagnosed cancer in Spain in 2022, with 34,750 new cases (1). Hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative is the most common subtype of BC, accounting for around 60% of cases (2).
Although only 6% of patients present metastatic breast cancer (MBC) at the time of diagnosis (3), many patients initially diagnosed at stages I-III will have advanced disease throughout the course of their disease (4). In this setting, the therapeutic goal is palliative, and treatment is directed according to HR and HER2 expression, previous medical records, and the menopausal status of the patient (5-7). Main clinical guidelines recommend cyclin-dependent kinase 4/6 inhibitors (iCDK) in combination with hormonal therapy as first-line treatment in postmenopausal women with HR+, HER2-negative advanced BC. Combination with an aromatase inhibitor (AI) is preferred unless the patient relapsed during or within 12 months of adjuvant treatment with an AI, in which case fulvestrant is the recommended option. Premenopausal women should be treated the same way as postmenopausal women, adding ovarian suppression or ablation to the treatment. A remarkable exception is patients with a life-threatening, rapid metastatic progression, in whom chemotherapy is indicated since it achieves a more significant organ response. After stabilisation, it would be indicated to interrupt chemotherapy and maintain HT (5-7).
ICDKs have been one of the major advances in the treatment of MBC in recent years. Through inhibition of CDK4/6, they reduce cell proliferation by blocking cell progression from G1 to S phase of the cell cycle. Palbociclib was the first to be authorised in Europe, with the indication of treatment of HR+ and HER2-negative locally advanced or MBC in combination with an AI or fulvestrant, the latter in the case of women who have received prior hormone therapy (8).
The phase III PALOMA-2 clinical trial (9, 10) was a multicenter, double-blind study that randomised 666 postmenopausal women with ER+ and HER2-negative locally advanced or MBC, not eligible for resection or curative radiotherapy who had not received prior systemic treatment for advanced disease, to receive either palbociclib+letrozole or placebo+letrozole.
In the first analysis, with a median follow-up of 23 months (9), the study already achieved its primary endpoint of PFS improvement. In the updated analysis with a median follow-up of 37.6 months (10), the median PFS were 27.6 (95% confidence interval (CI): 22.4-30.3) and 14.5 months (95%CI: 12.3-17.1), respectively; with a HR of 0.563 (95%CI: 0.461-0.687; p<0.000001) favoring the palbociclib + letrozole arm. Overall survival (OS) results were presented at the American Society of Clinical Oncology 2022 annual meeting, with no statistical OS benefit (11).
Regarding its tolerability, the most common adverse effect (AE) was neutropenia (81.8%) followed by leukopenia (40.3%), fatigue (39.6%), arthralgia (37.6%), nausea (37.2%) and alopecia (33.6%). 79.3% of patients had grade 3 or 4 AEs, the most frequent being neutropenia (69.1%). 12.2% required permanent treatment discontinuation because of AEs (9, 10).
Although real-world observational data on palbociclib as first-line treatment of HR+, HER2-negative locally advanced or MBC is increasing, there is a broad variability among the reported PFS and frequency of AE. None of these studies reports data on adherence (12-16).
In a systematic review published in 2016 (17), three studies which analysed adherence to oral antineoplastic therapy in patients with BC were identified. In these studies, adherence varied between 69% and 100%. In the mentioned review, the authors observed a consistent association between poor adherence and worse clinical outcomes, including a lesser response to therapy and higher mortality (17).
Once a drug is approved, it is administered to a heterogeneous group of patients with variable conditions, so extrapolating the data obtained in randomised clinical trials to clinical practice is challenging. Real-life data are essential to minimise this efficacy-effectiveness gap since they determine the effectiveness and tolerability of drugs in clinical practice, how other factors influence them, and also reveal the patterns of their use (18).
This study aimed to evaluate the real-world effectiveness and tolerability of palbociclib as first-line treatment of HR+, HER-negative locally advanced or MBC and how it compares with the results from the PALOMA-2 trial, as well as estimate adherence to palbociclib in this setting.
METHODS
We conducted a retrospective, unicenter, longitudinal, observational study at a tertiary Hospital. All adult women with HR+ and HER2-negative locally advanced or MBC who started first-line treatment with palbociclib at Hospital Universitario Ramón y Cajal (Madrid, Spain) between January 2018 and August 2019 were included. Individual patient data were manually collected from electronic clinical records (ECR, HCIS®) and the Pharmacy dispensing system (Hospiwin®). The study received approval by the Hospital Universitario Ramón y Cajal Drug Research Ethics Committee (study ID 149/21).
The primary effectiveness outcome was PFS, defined as the time from the first dose of palbociclib to disease progression or death according to Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 criteria (18). A stratified analysis of PFS was also performed according to exposure to palbociclib (a relative dose intensity cut-off point of 85% was established) and inclusion and exclusion criteria for the PALOMA-2 trial. Secondary effectiveness outcomes were overall survival (OS), overall response rate (ORR), duration of response, and clinical benefit rate (CBR), defined as achieving a complete response (CR), partial response (PR) or stable disease for ≥24 weeks.
Safety outcomes were frequency and grade of AE according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (19) and frequency, reasons and time to dose reductions, cycle delays and permanent treatment discontinuations. We also calculated relative dose intensity, defined as the proportion between the dose administered during the treatment period –assuming that the patient takes 100% of dispensed doses- and the standard palbociclib regimen (125 mg once daily for three weeks, followed by one week off). Data on deaths potentially related to palbociclib were also collected.
Adherence was determined through dispensing records, using the proportion of days covered (PDC), defined as the proportion of days in which the patient has enough medication to take 100% of prescribed doses. It assumes that a patient cannot take medication that is not dispensed to her, and she correctly takes the dispensed doses (20). A patient was considered adherent when PDC was ≥80%.
Demographic and clinical variables were collected and presented similarly to the PALOMA-2 scientific paper. It is worth clarifying that multiple lesions in the same organ were counted as one metastatic site.
Categorical variables were analysed according to their absolute frequency. Quantitative variables were defined by the median and interquartile range (IR). All CI were defined as 95%. For comparison with clinical trial results, we used the aggregated results of PALOMA-2, published by Finn et al. (8, 9). The Kaplan Meier method was utilised to estimate PFS curves. Survival curves were graphically compared with PALOMA-2. Subgroups PFS curves comparison was performed using the log-rank test. SPSS Statistics® (v28) was utilised to perform the statistical analyses.
RESULTS
Patients’ demographic and clinical characteristics
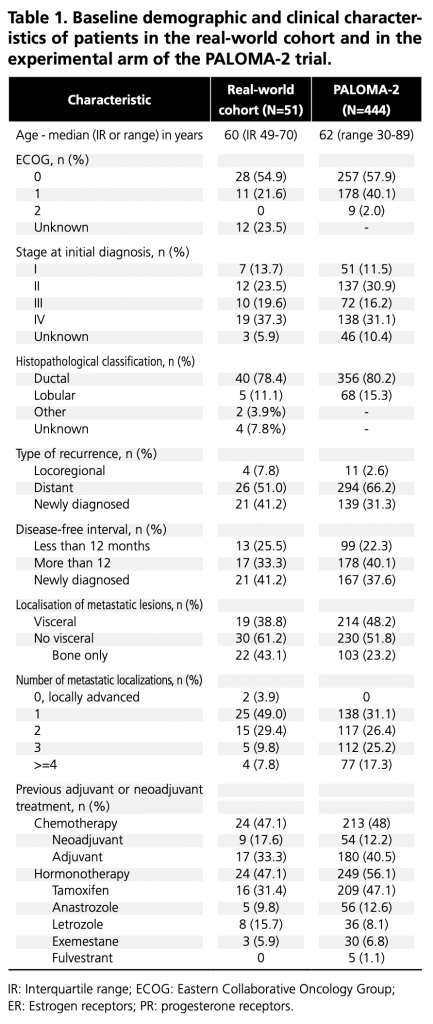
A total of 51 women were included, with a median follow-up of 21.7 months for the primary endpoint. Baseline demographic and clinical characteristics are compared with the PALOMA-2 experimental cohort (9) in Table 1. Six patients (11.8%) had relapsed during or within one year of completing adjuvant treatment with an AI, and 7 (13.7%) were premenopausal at the start of treatment; both were exclusion criteria for PALOMA-2.
IR: Interquartile range; ECOG: Eastern Collaborative Oncology Group; ER: Estrogen receptors; PR: progesterone receptors.
At the end of the follow-up, 10/31/2021, 15 (29.4%) patients continued to receive palbociclib. All patients started palbociclib standard dosing (125 mg daily for three weeks, followed by one week off), mainly combined with letrozole (78.4%). Palbociclib was prescribed in other combinations to a lesser extent: with fulvestrant (9.8%), tamoxifen + goserelin for subsequent switch to letrozole (7.8%), exemestane (2.0%), and tamoxifen alone (2.0%).
Effectiveness
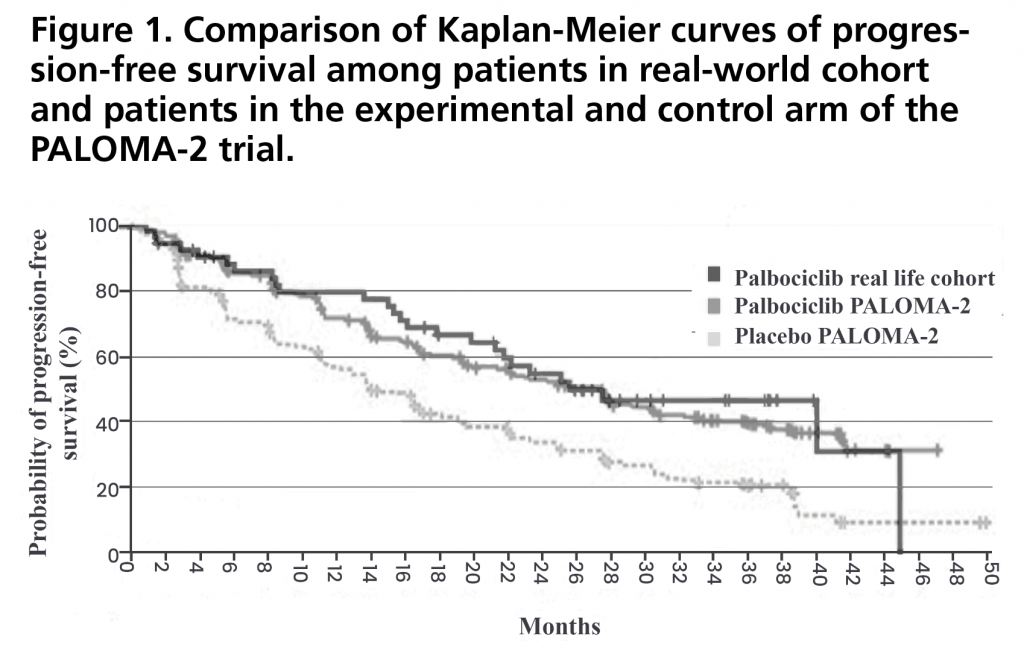
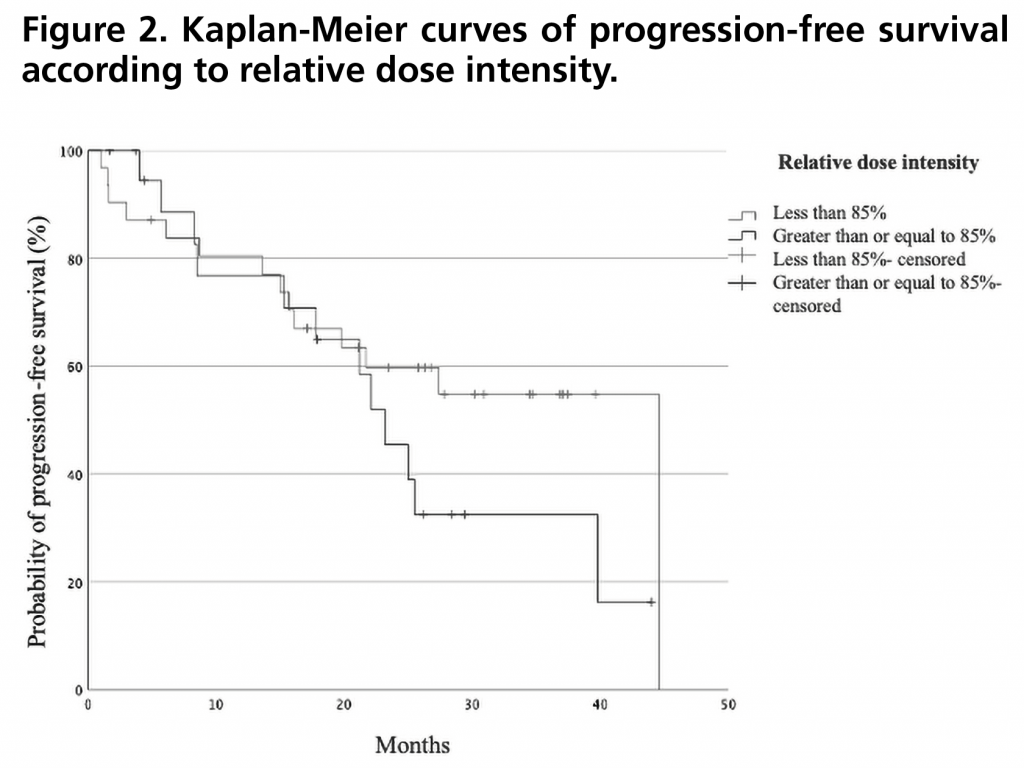
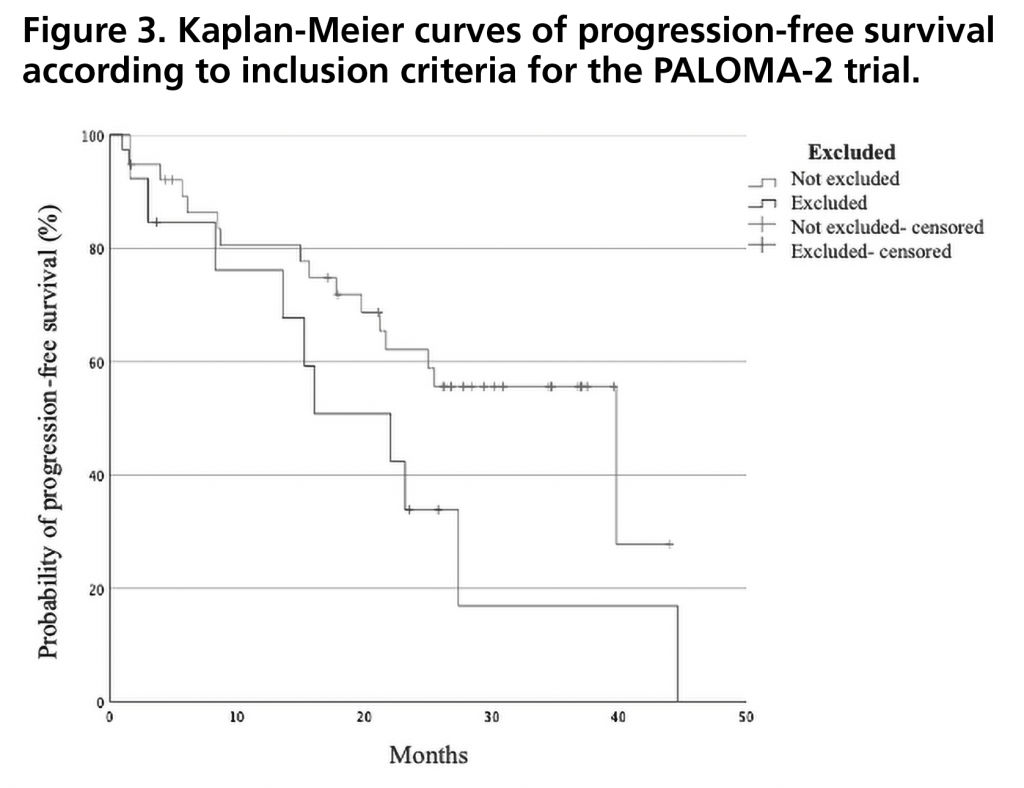
PFS was 27.4 months (95%CI: 15.5-35.5). Figure 1 shows the PFS curve compared to that obtained in the PALOMA-2 trial. PFS rate two years after initiation of treatment was 43% (95%CI: 29-57). There were no statistically significant differences in PFS when stratified by relative dose intensity, being 44.6 months (95%CI not estimable) for those who received a relative dose intensity <85% versus 23.2 months (95%CI 18.4-28.0) for those who received a dose ≥85% (p=0.257) (Figure 2). There was also no significant difference between patients who met the inclusion criteria and none of the exclusion criteria for the PALOMA-2 trial, and patients who would have been excluded from this trial obtaining a PFS of 39.8 months (95% CI 19.5-60.1) versus 22.1 months (95%CI: 10.9-33.3) (p=0.102) (Figure 3).
At the end of the follow-up, the number of events required to calculate the median OS had not occurred. The 2-year survival rate after initiation of treatment with palbociclib was 88% (95%CI: 79-97). ORR was 39% (95%CI: 25-53), with a median duration of response of 22.0 months (95%CI: not estimable). CBR was 82% (CI95%: 72-93).
Safety
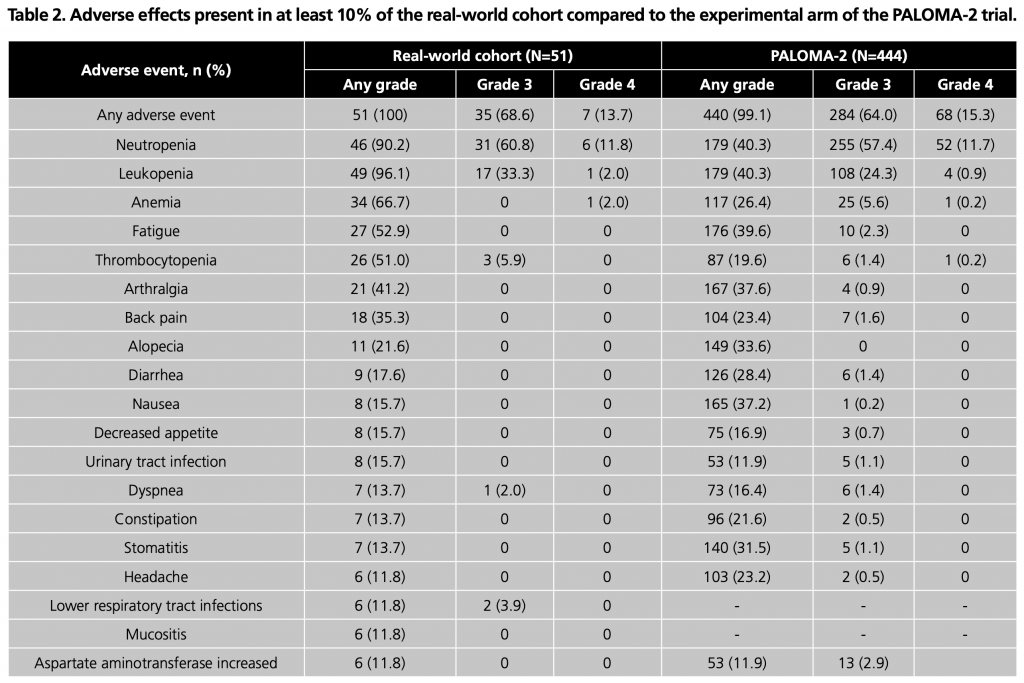
Regarding the safety profile of palbociclib, all patients experienced some AE, and 42 (82.3%) of them presented G3-4 AE. The most frequent G3-4 AE were neutropenia (72.5%), leukopenia (35.3%), thrombocytopenia (5.9%) and pneumonitis (5.9%). Despite the high incidence of neutropenia, a single patient (2.0%) developed febrile neutropenia, category G3. AE with a frequency greater than 10% and their grade are summarised in Table 2.
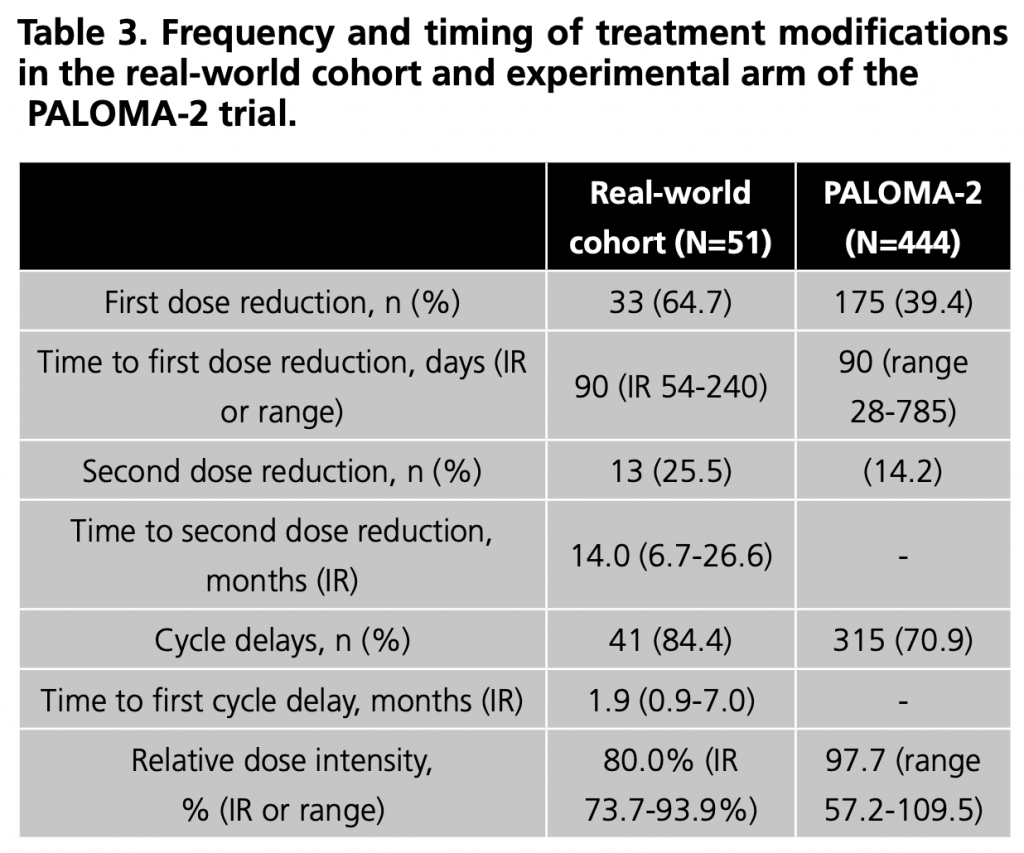
Regarding treatment modifications (Table 3), dose reductions were mainly due to neutropenia (69.7%), fatigue (9.1%) and mucositis (9.1%). In patients who required a second dose reduction, neutropenia (61.5%) was again the primary cause.
Patients who experienced at least one delay due to AE had a median of 3 delays (IR 2 – 5). Neutropenia (82.9%), fatigue (9.8%), thrombopenia (7.3%) and aspartate aminotransferase increase (7.3%) were the main reasons for cycle delays.
During follow-up, there were 36 (70.6%) permanent treatment discontinuations. The main reason was disease progression in 24 patients (47.1%). In contrast, 7 (13.7%) were discontinued due to AE, 3 (5.9%) due to loss of follow-up at our hospital, one (2.0%) due to negative HR expression in a metastatic liver specimen and another (2%) due to personal reasons of the patient. The main reasons for permanent treatment discontinuations due to adverse effects were neutropenia (42.9%), pneumonitis (28.6%), fatigue (14.3%) and progressive multifocal leukoencephalopathy (PML) (14.3%). Two deaths possibly related to treatment; were due to pneumonitis and PML.
Adherence
Median PDC was 99.4% (IR: 98.1-100%). All patients had adherence above ≥80% and were considered adherent.
DISCUSSION
Our cohort presents demographic and baseline clinical characteristics similar to those of the experimental cohort of the PALOMA-2 trial. Nonetheless, it included premenopausal patients and patients who had progressed during or in the year following discontinuation of adjuvant treatment with AI, both exclusion criteria in PALOMA-2. Regarding concomitant hormonotherapy, we included other combinations as first-line treatment of locally advanced MBC, with fulvestrant being the second most common after letrozole.
We obtained PFS results similar to PALOMA-2 (27.4 and 27.6 months, respectively). There is some variability in the effectiveness data obtained in other observational studies that seem to be due to heterogeneity, both in the size and characteristics of the populations studied and in the way the results are collected and expressed. García-Trevijano et al. (11) obtain a PFS for palbociclib as first-line treatment of 18.77 months (95%CI: 16.6-NE), a lower result probably influenced by the small sample size since only six patients received palbociclib as first-line treatment. Sampedro et al. (12) obtain a PFS of 22 months (95%CI 17.7-26.3%), also lower, possibly justified by older age, and a greater number of patients who received previous chemotherapy for localised disease or who presented with visceral involvement. The largest cohort (754 patients) reporting real-life median PFS data for palbociclib as first-line treatment for locally advanced or MBC is the study by Brufsky et al. (13). Using the Flatiron Health Analytics database, they report a PFS of 20.0 months (95% CI 17.3-23.3). Some factors that may justify a lower PFS are older age, patients with poorer functional status (6% of patients had an ECOG ≥2), and the greater variability in care practice given the large number of centres and, therefore, professionals involved. The relatively high number of missing or unknown data should also be outlined.
Regarding the safety profile of palbociclib, despite a similar incidence of G3-4 AE and permanent discontinuations due to AEs, we observe more dose delays and dose reductions due to AE compared to PALOMA-2, resulting in a lower median relative dose intensity. Wilkie et al. (14) and Beachler et al. (15) also present a higher frequency of dose reductions and delays compared to PALOMA-2, although in both cases, their incidence is lower than in our cohort. These differences could respond to different management of AE in clinical practice, which could be more conservative given the limited experience in the use of these drugs, or they could be related to the persistence of AE over time and the aim of a higher quality of life in patients with a palliative treatment objective. We do not know if including patients who would not be eligible for the PALOMA-2 trial could influence this. However, our results and those obtained by Wilkie et al. -SLP 26.4 months- (14) and Beachler et al. (15) -SLP at two years of 64.9%-show that a lower overall drug exposure does not seem to compromise treatment effectiveness. Also, we did not find differences in PFS according to relative dose intensity, and neither Wilkie et al., who analysed whether patients obtained different PFS results depending on the dose finally tolerated.
Remarkably, two deaths were considered potentially related to palbociclib. One was caused by pneumonitis, for which the FDA issued a safety alert because of severe cases and deaths related to palbociclib (21). PML caused the other, a rare pathology that occurs almost exclusively in immunosuppressed individuals and is related to the use of immunomodulatory and immunosuppressive drugs, including other antineoplastic drugs and with malignancy, although mainly haematological (22), so there is some biological plausibility that the development of PML could be related to palbociclib-induced neutropenia and leukopenia. As a contributing factor, the patient was diagnosed with systemic sclerosis and was treated with cyclophosphamide nine months before the onset of PML.
Adherence to treatment with palbociclib was extremely high. There is high variability in the adherence rates reported in oncology patients, which may be justified by the different populations and study drugs and the different measurement instruments used. Partridge et al. (23), in a study in patients with localised stage BC treated with capecitabine, obtained an adherence rate of 78%, with up to 25% of patients presenting adherence of less than 80%. The measurement method used was electronic monitoring devices, which are more accurate than dispensing registers. Therefore, adherence may have been overestimated in our study. However, it would not be possible to discard that greater treatment tolerability could justify better adherence in our study, the influence of a different clinical scenario (early and advanced disease, respectively), and the age of the patients since Partridge et al. only included patients over 65 years of age.
Regarding the study’s main limitations, it is worth highlighting those inherent to retrospective studies, such as the inability to recover past data or insufficiently documented events. This may have influenced the type and number of AE reported since documentation of patient-reported adverse events, such as diarrhoea, nausea, or vomiting, may be underrepresented in comparison with laboratory results automatically registered in the ECR. Although there is a high number of ECOG statuses missing, those patients likely had a good performance status (ECOG 0-1) as the hospital treatment protocol only allowed its use in that setting. The small sample size corresponding to a single-centre may influence the external validity of the results. Median follow-up, although sufficient to obtain results concerning PFS, did not allow conclusions to be drawn about the effect of palbociclib on OS. It is also worth noting that up to 29.4% of the sample was still receiving treatment at the end of follow-up, which translates into a high number of patients censored towards the end of the PFS curve, preventing us from observing whether there is a proportion of patients with high response in terms of PFS to palbociclib. Finally, another limitation would be adherence measurement by a single measurement method. It is considered that an optimal assessment of adherence should combine at least two methods in order to overcome their respective limitations, preferably dispensing records together with an adherence questionnaire (14). As this was a retrospective study, it was not possible to assess adherence using questionnaires, which could have allowed the detection of qualitative adherence problems (e.g., difficulty swallowing, management of adverse effects) that cannot be detected using dispensing records.
As strengths, to the authors’ knowledge, this study would present the largest sample size and the most extended follow-up published in the Spanish population to date. Second, it encompasses a homogeneous population since all patients received palbociclib as first-line treatment for HR+ and HER2-negative locally advanced or MBC. Finally, by including all patients who were dispensed at least one dose of palbociclib, selection biases are minimised, and, therefore, it faithfully reflects the real-life experience of our centre with this drug as first-line treatment.
Conclusion
This study’s results add to published literature on real-life experience with palbociclib as first-line treatment of HR+ and HER2-negative locally advanced or MBC, providing evidence that the benefit demonstrated in randomised clinical trials translates to routine clinical practice.
Palbociclib’s safety profile in clinical practice was generally consistent with the results of PALOMA-2 and in line with published observational studies, including severe cases of pneumonitis. Despite differences in treatment modifications, they did not appear to affect treatment effectiveness.
Adherence to palbociclib treatment was high, reflecting its good tolerability and acceptability among patients.
References
- Sociedad Española de Oncología Médica, (SEOM). Las cifras del cáncer en España. Sociedad Española de Oncología Médica (SEOM) 2022. Available: https://seom.org/images/LAS_CIFRAS_DEL_CANCER_EN_ESPANA_2022.pdf. [Accessed Mar 2022].
- Parise CA, Bauer KR, Brown MM, et al. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999- 2004. Breast J 2009;15:593-602.
- National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer. 2022; Available: https://seer.cancer.gov/statfacts/html/breast.html. [Accessed Apr 2022].
- Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. The New England journal of medicine 2017 Nov 9; 377(19):1836-1846.
- National Cancer Comprehensive Network. Breast Cancer version 2.2022. 2022; Available: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. [Accessed Apr 2022].
- Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol 2021;32:1475-95.
- Burstein HJ, Somerfield MR, Barton DL, et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: ASCO Guideline Update. J Clin Oncol 2021;39:3959-3977.
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica Ibrance 100 mg cápsulas duras. 2016; Available: https://cima.aemps.es/cima/dochtml/ft/1161147003/FT_1161147003.html#10-fecha-de- la-revisi-n-del-texto. [Accessed Mar 2022].
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-1936.
- Rugo H, Finn R, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019;174:719-729.
- Pfizer. Pfizer Announces Overall Survival Results from Phase 3 PALOMA-2 Trial of IBRANCE® (palbociclib) for the First-Line Treatment of ER+, HER2- Metastatic Breast Cancer. 2022; Available: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-overall-survival-results-phase-3-paloma-2 [Accessed Jun 2022].
- García-Trevijano Cabetas M, Lucena Martínez P, Jiménez Nácher I. Real-world experience of palbociclib and ribociclib: novel oral therapy in metastatic breast cancer. Int J Clin Pharm 2021;43(4):893-899
- Sampedro T, Pampin R, Barbazan J, et al. Observational real-world data with palbociclib associated to hormone therapy for advanced breast carcinoma. Farm Hosp 2021;45(6):329-334.
- Brufsky A, Liu X, Li B, et al. Real-World Tumor Response of Palbociclib Plus Letrozole Versus Letrozole for Metastatic Breast Cancer in US Clinical Practice. Targ Oncol 2021;16(5):601-611.
- Wilkie J, Schickli MA, et al. Progression-Free Survival for Real-World Use of Palbociclib in Hormone Receptor-Positive Metastatic Breast Cancer. Clin Breast Cancer 2020;20(1):33-40.
- Beachler DC, de Luise C, Jamal-Allial A, et al. Real-world safety of palbociclib in breast cancer patients in the United States: a new-user cohort study. BMC Cancer 2021;21(1):97.
- Greer JA, Amoyal N, Nisotel L, et al. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist 2016;21:354-76.
- Lamas Díaz MJ, Zarra Ferro I. Investigación y evaluación de resultados en salud (datos de vida real). En: Martínez Sesmero JM, Calleja Hernández MÁ, editores. Manual de Investigación e Innovación para residentes en Farmacia Hospitalaria. Madrid; 2017: 41-59.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2008;45:228-247.
- National Cancer Institute. National Cancer Institute Common Terminology Criteria for Adverse Events, v5.0. 2017; Available: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_qui ck_reference_5x7.pdf. [Accessed Mar 2022].
- Ibarra Barrueta O, Morillo Verdugo R. Lo que debes saber sobre la adherencia al tratamiento. Madrid: Sociedad Española de Farmacia Hospitalaria; 2017.
- Food and Drugs Administration. La FDA advierte sobre la inflamación pulmonar poco frecuente, pero grave con Ibrance, Kisqali y Verzenio para el cáncer de mama. 2019; Available: https://www.fda.gov/drugs/drug-safety-and-availability/la-fda-advierte- sobre-la-inflamacion-pulmonar-poco-frecuente-pero-grave-con-ibrance-kisqali-y. [Accessed Mar 2022].
- Koralnik I. Progressive multifocal leukoencephalopathy (PML): Epidemiology, clinical manifestations, and diagnosis. En: Ted. W. Post, editor. UpToDate. Waltham, MA; 2021. Available at: https://www.uptodate.com/. [Accessed Dec 2022].
- Partridge AH, Archer L, Hudis C, et al. Adherence and Persistence With Oral Adjuvant Chemotherapy in Older Women With Early-Stage Breast Cancer in CALGB 49907: Adherence Companion Study 60104. J Clin Oncol 2010;28:2418-22.