Sánchez Ruiz A, Oya Álvarez de Morales B, Muñoz Cid CL
- Hospital Pharmacy Department, Universitary Hospital of Jaen, Spain
Fecha de recepción: 11/05/2023 – Fecha de aceptación: 15/06/2023
Correspondencia: Andrés Sánchez Ruiz · Hospital Universitario de Jaén. Av. del Ejército Español, 10, 23007 Jaén, Spain · Correo: andres.sanchez.ruiz2@gmail.com
____
Alopecia areata is an autoimmune disease that occurs with a chronic, recurrent inflammatory process that weakens the hair follicles, causing hair loss. Janus kinase (JAK) inhibitors have been shown to be effective orally in patients with moderate-severe AA. We performed an electronic search and a case series study analysing effectiveness of tofacitinib off-label use in alopecia areata in our patients. 2 RCTs were included in our review (baricitinib pivotal studies), 1 network meta-analysis, 3 systematic review/metanalysis and 2 open comparative studies. According to the results obtained in the studies and published reviews, JAK-kinase inhibitors appear to induce hair regrowth in about half of patients, specially according to the abundant tofacitinib available evidence. The adverse effects founded are those expected from this class of drugs.
Keywords: Alopecia, tofacitinib, baricitinib, evaluación, eficacia
Evidencia del uso off-label de tofacitinib en alopecia areata
La alopecia areata (AA) es una enfermedad autoinmune que cursa con un proceso inflamatorio crónico y recurrente que debilita los folículos pilosos, provocando la caída del cabello. Se ha demostrado que los inhibidores de la cinasa de Janus (JAK) son efectivos por vía oral en pacientes con AA moderada a grave. Realizamos una búsqueda electrónica y un estudio de serie de casos que analizó la efectividad del uso off-label de tofacitinib en alopecia areata en nuestros pacientes. En nuestra revisión se incluyeron 2 ECA (estudios pivotales de baricitinib), 1 metanálisis en red, 3 revisiones sistemáticas/metanálisis y 2 estudios comparativos abiertos. Según los resultados obtenidos en los estudios y revisiones publicadas, los inhibidores de la JAK-quinasa parecen inducir el crecimiento del cabello en aproximadamente la mitad de los pacientes según la abundante evidencia disponible sobre tofacitinib. Los efectos adversos encontrados son los esperados de esta clase de fármacos.
Palabras clave: Alopecia, tofacitinib, baricitinib, evaluación, eficacia
____
Introduction
Alopecia areata (AA) is an autoimmune disease that occurs with a chronic, recurrent inflammatory process that weakens the hair follicles, causing hair loss, especially on the scalp. 1 A worldwide prevalence of 1 per 1,000 people is estimated, with a lifetime risk of approximately 2%.2,3 AA can appear in any age range and does not distinguish between sexes. 2
There are three types of AA: AA patchy, characterized by appearance of round and oval patches on the head or on other places of the body. AA totalis, which presents with complete loss of scalp hair. Finally, AA universalis, when there is total loss of hair on the entire body, face and scalp. 4
Pathogenesis is not fully known yet. In patients with AA, hair follicles in the growing phase prematurely enter the non-proliferative involution and resting phases, causing hair loss and growth inhibition. 5 In mice, there is an increase in Interleukin (IL)-15 in the hair follicles and this causes the recruitment and activation of CD8(+) NKG2D (+) cytotoxic T cells, which, in turn, produce interferon- γ(INF-γ) and IL-15 further activating the epithelium of the hair follicle. Cell signaling occurs through the Janus kinase (JAK) family of enzymes.6
Janus kinase (JAK) inhibitors have been shown to be effective orally in patients with moderate-severe AA7. The most studied in this pathology have been Tofacitinib, Baricitinib and Ruxolitinib.
Methods
An electronic search was performed in Medline and Embase on february, 2023, according to PRISMA guidelines. The terms used were “alopecia” AND (“tofacitinib” OR “JAK-kinase” OR “baricitnib” OR “ruxolitinib”). We included studies of AA patients treated with iJAK drugs and other oral drugs used in AA. Studies consisting of abstracts or case series with fewer than 10 participants were excluded.
We also reflected the experience in our hospital with the use of tofacitinib off-label in AA. All patients treated for at least one month with tofacitinib were selected. The following variables were collected: sex, date of birth, type of AA, number of dispensings, duration of active treatment in months, whether or not the patient continues to be active, reason for treatment suspension, Severity of Alopecia Tool (SALT) at 36 weeks and adverse reactions. Data were collected from the pharmacy electronic system and from medical records.
A comparative table with the different studies was designed to try to make the comparison more visual (table 1). The following were compiled in this table: number of patients included in the study, date, drugs studied and comparator, main variable, results and adverse reactions.
Results
2 RCTs were included in our review, 1 network meta-analysis, 3 systematic review/metanalysis and 2 open comparative studies.
The available RCTs were the phase 3 BRAVE AA1 and BRAVE AA2 studies8,9, giving approval to baricitinib 4 mg and 2 mg, respectively, versus placebo. The main variable used in these studies was SALT<20 at week 36, resulting 38.8% of the patients in BRAVE AA1 and 22.8% in BRAVE AA2. The main adverse reactions were acne and elevated levels of creatine kinase, LDL and HDL. Two other studies use the SALT 50 variable as the main variable, as can be seen in the table below. The most representative study in terms of number of patients was the network meta-analysis10, with a sample of 3209 patients. It included studies with a total of 49 different treatment options and the results were unified in the variable OR of treatment success rate (>50%), in which tofacitinib obtained an OR of 6.93 (1.1-44.7, p=0.42).
The 3 systematic reviews11,12,13 identified include around 300 patients each. Two of them included, in addition to tofacitinib, other iJAKs. They differed in the main outcome used, as we can see in Table 1. if we assimilate the main outcomes used in these studies; good response 50-100%, good/complete hair regrowth rate and SALT50, the results obtained in percentage of success are between 45.7% and 66%.
Table 1 also shows two other studies14,15 that include a smaller number of patients, as well as the results of the patients analyzed in our hospital and who were treated with tofacitinib.
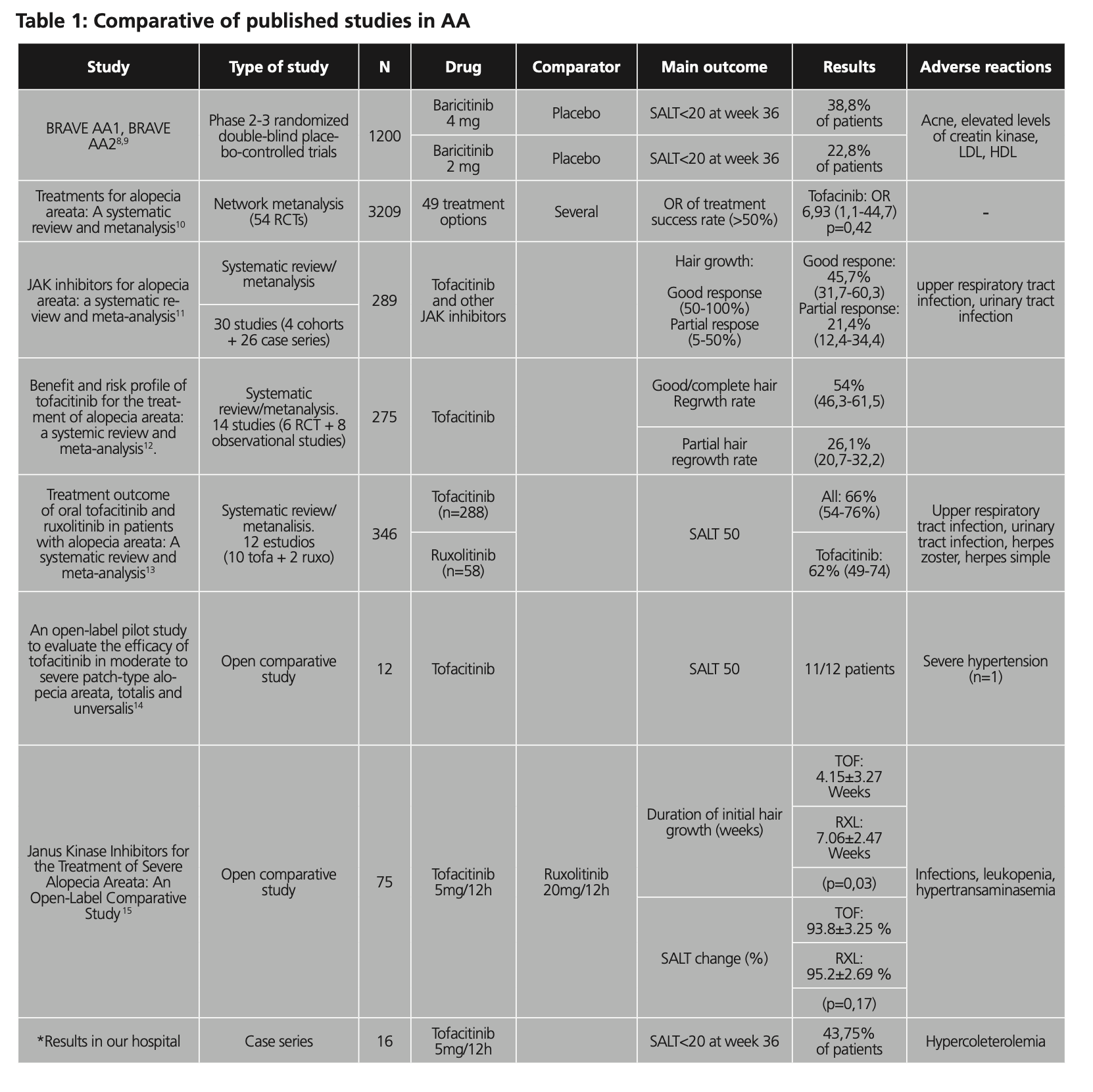
In general, the most common adverse reactions reported in the different studies analyzed in which iJAK are used are those related to laboratory abnormalities such as hypertransaminemia or elevated creatine kinase and upper respiratory tract and urinary tract infections, as well as viral infections Herpes zoster-like.
On the other hand, we evaluated the efficacy and safety of tofacitinib in the off-label treatment of alopecia areata in adolescents and adults treated at our hospital. The study included 16 patients treated with tofacitinib 5mg/12h, 8 of them women and 8 men, with a mean age of 35 years (median 34 years). The most common form of diagnosed AA was universal (n=9), followed by alopecia diffuse (n=3). 12 patients (75%) were still on treatment at the time of conduct of the study. The median duration of treatment was 30.80 (1.02-53.02) months for the total number of patients, and 20.08 months in the case of patients who discontinued the drug. However, the median duration of treatment was 46 months in patients who achieved total repopulation, all of them active at the time of the study. The efficacy measured according to patients who achieved SALT20 (80% improvement) was 43.75% of patients. In two cases, follow-up was lost. Regarding toxicity, it was declared hypercholesterolemia/hypertriglyceridemia in a total of 4 patients (25%), one of them leading to discontinuation. Another patient failed after first month of treatment for an unspecified intolerance.
Discussion
According to the results obtained in the studies and published reviews, JAK-kinase inhibitors appear to induce hair regrowth in alopecia areata (AA). Although RCTs are only available for the case of baricitinib, the evidence for tofacitinib is abundant, as can be seen in the available meta-analyses. There is heterogeneity in terms of the selected population, treatment regimens used, and efficacy variables chosen among the different studies. However, in general the results seem to be similar, with significant percentages of patients benefiting to a greater or lesser extent from treatment with JAK-kinase inhibitors. This fact is also reflected in our small sample of patients, in which slightly less than half of them presented complete or almost complete repopulation of hair. The adverse effects found in the evidence analyzed are those expected from this class of drugs, highlighting analytical abnormalities (creatine kinase, transaminases) and infections (upper respiratory tract, Herpes-Zoster).
Conflictos de intereses: Los autores declaran no tener conflictos de intereses.
Bibliography
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403. doi:10.2147/CCID.S53985.
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. 2018;78(1):1-12. doi:10.1016/j.jaad.2017.04.1141
- Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134(4):1141-1142. doi:10.1038/jid.2013.464
- Guo L, Feng S, Sun B, Jiang X, Liu Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020 Jan;34(1):192-201. doi: 10.1111/jdv.15937.
- Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. doi:10.1038/nrdp.2017.11
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043-1049. doi:10.1038/nm.3645
- Almutairi N, Nour TM, Hussain NH. Janus Kinase Inhibitors for the Treatment of Severe Alopecia Areata: An Open-Label Comparative Study. Dermatology. 2019;235(2):130-136. doi:10.1159/000494613
- King B, Ko J, Forman S et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Aad Dermatol 2021; 85:847–53.
- King B, Ohyama M, Kwon O et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med 2022; 5:1687–99.
- Fukumoto T, Fukumoto R, Magno E, Oka M, Nishigori C, Horita N. Treatments for alopecia areata: A systematic review and network meta‐analysis. Dermatol Ther. 2021;34(3). doi:10.1111/dth.14916.
- Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta‐analysis. J Eur Acad Dermatol Venereol. 2019;33(5):850-856. doi:10.1111/jdv.15489
- Guo L, Feng S, Sun B, Jiang X, Liu Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: a systemic review and meta‐analysis. J Eur Acad Dermatol Venereol. 2019;34(1):192-201. doi:10.1111/jdv.15937
- Yu DA, Kim YE, Kwon O, Park H. Treatment outcome of oral tofacitinib and ruxolitinib in patients with alopecia areata: A systematic review and meta-analysis. Indian J Dermatol Venereol Leprol. 2021;87:621-627. doi:10.25259/ijdvl_975_19
- Jabbari A, Sansaricq F, Cerise J et al. An Open-Label Pilot Study to Evaluate the Efficacy of Tofacitinib in Moderate to Severe Patch-Type Alopecia Areata, Totalis, and Universalis. J Investig Dermatol. 2018;138(7):1539-1545. doi:10.1016/j.jid.2018.01.032
- Almutairi N, Nour TM, Hussain NH. Janus Kinase Inhibitors for the Treatment of Severe Alopecia Areata: An Open-Label Comparative Study. Dermatology. 2018;235(2):130-136. doi:10.1159/000494613
____
