Campos Fernández de Sevilla MA1, Pérez Parra F2, Gallego Úbeda M1, Tutau Gómez F1
1 Pharmacy Department
2 Neurology Department
Hospital Universitario del Henares. Madrid (Spain)
Fecha de recepción: 20/07/2020 – Fecha de aceptación: 14/09/2020
Correspondencia: María Ángeles Campos Fernández de Sevilla – Hospital Universitario del Henares (Pharmacy Department) – Avda. Marie Curie, s/n – 28822 Coslada, Madrid (Spain)
mangeles.campos@salud.madrid.org
____
SUMMARY
Objective: To report a case of local reaction to natalizumab extravasation and its resolution supported by telepharmacy.
Case summary: 46-year-old woman, with Raynaud’s sindrome, inflammatory arthralgia and relapsing-remitting multiple sclerosis recieved an intravenous dose of 300 mg of natalizumab. Twenty-four hours after the infusion, the patient contacted the Pharmacy Department by email due to an inflammatory, erythematous, and scaly painless reaction in the right hand. The patient provided pictures of the local reaction, which is diagnosed as an extravasation reaction by natalizumab. To avoid the hospital visit due to COVID-19 pandemic, medical and pharmaceutical teleconsultation was performed and topical corticosteroids and oral antibiotic were prescribed.
The extravasation injury resolved favorably after five days of treatment, without visiting the hospital.
Discussion: Extravasated natalizumab is a potential irritant. The local reaction observed can be attributed to the pH of natalizumab solution, incorrect venipunture technique and risk factors present in the patient. The knowledge of the patient’s risk factors is key for the prevention.
Telepharmacy allowed assessing the evolution of the injury in the patient’s home by a multidisciplinary team, avoiding visits to the hospital during COVID-19 pandemic.
Key words: Extravasation, natalizumab, telepharmacy, case report.
Describir un caso de reacción local por extravasación de natalizumab y su resolución mediante telefarmacia
RESUMEN
Objetivo: Describir un caso de reacción local por extravasación de natalizumab y su resolución mediante telefarmacia.
Descripción del caso: Mujer de 46 años, con síndrome de Raynaud, artralgia inflamatoria y esclerosis múltiple recurrente-remitente, recibió una dosis intravenosa de 300 mg de natalizumab. Veinticuatro horas después de la infusión, el paciente contactó con el Servicio de Farmacia del hospital mediante correo electrónico para notificar una reacción inflamatoria, eritematosa y no dolorosa en el lugar de la infusión intravenosa de natalizumab. La paciente proporcionó imágenes de la reacción local, diagnosticándose como una reacción de extravasación por natalizumab. Para evitar la visita al hospital de la paciente durante la situación de pandemia por COVID-19, se realizó teleconsulta médica y farmacéutica, prescribiéndose corticoides tópicos y antibióticos orales. La lesión se resolvió favorablemente mediante seguimiento por teleconsulta después de cinco días de tratamiento, evitando visitas de la paciente al hospital.
Discusión: Natalizumab es un potencial irritante. La reacción local de extravasación observada puede atribuirse al pH de la solución de natalizumab, la técnica incorrecta de venopunción y a los factores de riesgo presentes en nuestra paciente. El conocimiento de estos factores de riesgo es clave para su prevención. La telefarmacia permitió que un equipo multidisciplinar tratara y monitorizara la evolución de la lesión en el domicilio del paciente, evitando visitas al hospital durante la pandemia por COVID-19.
Palabras clave: Extravasación, natalizumab, telefarmacia, caso clínico.
____
INTRODUCTION
On March 11, 2020, the World Health Organization (WHO) declared a state of pandemic for COVID-19. In this context of global health crisis, many hospital pharmacy deparment in Spain have immediately launched telepharmacy processes to provide pharmaceutical care program for outpatients1.
Telepharmacy is an information and communication technology (ICT) that, among other advantages, makes possible the pharmacotherapeutic care program at the patient’s home and the coordination between the different health professionals2. Therefore, this tool could be especially useful in the prevention of COVID-19 in the most vulnerable patients, such as patients in treatment with natalizumab, a powerful immunosuppressive drug for the treatment of highly active forms of relapsing-remitting multiple sclerosis (RR-MS)3.
The aim of this study is to describe the case of a patient with an extravasation reaction to natalizumab, that was successfully managed through the use of telepharmacy.
DESCRIPTION OF THE CASE
46-year-old woman, with Raynaud’s syndrome and inflammatory arthralgia. In 2010, she was diagnosed with RR-MS. She started treatment with interferon-1a weekly, which had to be suspended due to disease progression in March 2015. After that, the patient received oral dimethyl fumarate 240mg/12h. In June 2017, intravenous natalizumab 300 mg every 4 weeks treatment was started, after suspension of dimethyl fumarate due to prolonged lymphopenia.
At that time, the patient presented a disability index of 4, according to the Expanded State of Disability Scale (EDSS), severe tactile hypoesthesia in the right arm and profound sensitivity impairment, among other neurological disorders derived from her demyelinating disease.
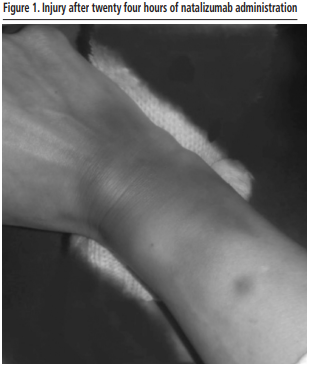
On March 14, 2020, the government of Spain declared a state of alarm for pandemic COVID-19. Therefore, the patient is teleconsulted to recommend home confinement, due to her immunosuppression situation derived from treatment with natalizumab. On April 15, the patient goes to the hospital, to receive a new intravenous dose of natalizumab. The infusion of the treatment was carried out without major incidents.
The patient only reported pain at the time of peripheral venous catheter insertion. Twenty-four hours after the infusion, the patient contacted the pharmacy department by email due to an inflammatory, erythematous, and scaly painless reaction in the right hand. The patient provided pictures of the local reaction, which is diagnosed as an extravasation reaction by natalizumab (Figure 1).
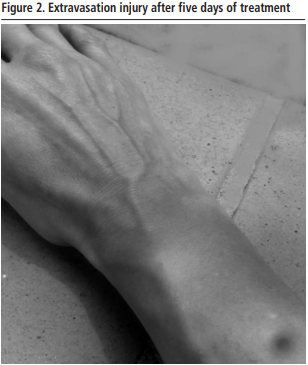
To avoid the hospital visit,medical and pharmaceutical teleconsultation was performed and topical corticosteroids (methylprednisolone 0.1%, one daily application) and oral antibiotic (amoxicillin/clavulanic acid 875/126 mg every 8 hours for 7 days) were prescribed. The evolution of the injury was followed by daily pharmaceutical teleconsultation with the support of pictures sent by e-mail to the pharmacy department. The extravasation injury resolved favorably after 5 days of treatment, without the need for admission or visit to the hospital (Figure 2).
This case has been reported to the National Health Authorities.
DISCUSSION
To date, there are no published cases of extravasation of natalizumab at the site of venous catheter insertion, so our case could be of particular interest. The manufacturer’s technical information on natalizumab indicates that it is non-toxic to the skin and is unknown to have a venous or skin irritant effect3. However, the local reaction observed in our patient demonstrates the local toxic effect of the extravasation of this drug.
There are several mechanisms and factors that could contribute to the appearance of tissue toxicity after extravasation of a drug4. The physicochemical properties of the drug may be an important factor for toxicity to occur after extravasation. Changes in pH affect celular and tisular homeostasis. Acidic and basic drugs mediate toxicity typically when the pH of the solution is very different from the physiological pH of human plasma range from 7.38 to 7.425. The pH of the natalizumab solution is 6.13, which could explain the toxicity observed in our patient.
The acidic compound can produce vasoconstriction, edema, disintegration, and ulceration of the skin. The severity of tissue damage depends on duration of exposure, the pH of the drug, and the titratable reserve (the theoretical amount o fan exogenous neutralizing substance neccessary to get back to the physiological pH)4.
There are no specific treatment recommendations in case of extravasation of natalizumab6, so the actions must be assesed on case-by-case bases, using a multidisciplinary approach4. In our case, the intervention of the pharmacist, neurologist and dermatologist was necessary. Neither ulceration nor necrosis appeared in our patient’s skin. Therefore, it was successfully managed with the application of topical corticosteroids and oral antibiotic treatment at home.
Besides, the patient presented Raynaud’s syndrome, neuropathy and hypoesthesia (difficulty in feeling pain) that could have also contributed to the toxicity produced by the extravasation of natalizumab. Some patients with multiple sclerosis are at risk because they are less sensitive to pain or irritation, which prevents them from noticing the toxicity of the drug7. So, the prevention is key to minimize the risk of extravasation in these patients. General prevention techniques to avoid these accidents include: correct choice of the site of injection; correct venipuncture techique performed by experienced personnel; test of vein integrity before administration of natalizumab; appropriate drug dilution and infusion time; regular inspection of the injection site during and up to one hour after the infusion for signs of infiltration of infused fluids and phlebitis; and close monitoring of patency of the line during administrations4,6.
Finally, it is the first published case of management of extravasation of natalizumab by telepharmacy. In our case, the treatment measures adopted and the monitoring of the evolution of the extravasation injury by teleconsultation allowed the resolution of the injury safely and effectively. The patient’s visit to the hospital was not necessary, thanks to the coordination between doctors and pharmacists, and close dayly monitoring until the symptoms disappear with the help of ICT.
Conflicts of interest: The authors declare that they have no conflict of interest.
____
BIBLIOGRAPHY
1. Morillo-Verdugo R, Margusino-Framiñán L, Monte-Boquet E, Morell-Baladrón A, Barreda-Hernández D, Rey-Piñeiro XM, et al. Spanish Society of Hospital Pharmacy Position Statement on Telepharmacy: Recommendations for its implementation and development. Farm Hosp. 2020 Jul 1;44(4):174-81.
2. Baldoni S, Amenta F, Ricci G. Telepharmacy Services : Present Status and Future Perspectives: A Review. 2019;1-12.
3. Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica de natalizumab [Internet]. Madrid: Centro de Información de Medicamentos 2020 [consultado 18/07/2020]. Disponible en: https://cima.aemps.es/cima/ pdfs/ft/06346001/FT_06346001.pdf.
4. David V, Christou N, Etienne P, Almeida M, Roux A, Taibi A, et al. Extravasation of Noncytotoxic Drugs. Ann Pharmacother. 2020 Aug;54(8):804-14.
5. Gil ME, Mateu J. Treatment of extravasation from parenteral nutrition solution. Ann Pharmacother [Internet]. 1998 Jan;32(1):51-5.
6. Leary SO, Beavin J, Bishop C, Capolino L, Greinel E, Hudson E. Practical Guidelines for Administering Natalizumab: A Nursing Perspective. 2007;1-8.
7. Scherder RJ, Kant N, Wolf ET, Pijnenburg BCM, Scherder EJA. Sensory Function and Chronic Pain in Multiple Sclerosis. Pain Res Manag. 2018;2018:1924174.
____
