Rev. OFIL 2017, 27;1:88-90
Fecha de recepción: 07/05/2016 – Fecha de aceptación: 31/10/2016
____
Toscano-Guzmán MD, Santos-Rubio MD, Poyatos-Ruiz LL, Gil-Navarro MV
Hospital Universitario Virgen del Rocío. Sevilla (España)
____
Correspondencia:
María Dolores Toscano Guzmán
Hospital Universitario Virgen del Rocío
C/Manuel Siurot, s/n
41013 Sevilla
Correo electrónico: maritogu@hotmail.com
____
SUMMARY
Case: 36 year-old male with body surface area of 2.44 m2, diagnosed with osteosarcoma, treated with chemotherapeutic scheme based on methotrexate, cisplatin/ifosfamide and adriamycin. Methotrexate was not set according to the existing recommendations of limiting the total dose to 20 g or, adjusting it to 2 m2, but 29.3 g (12 g/m2) was prescribed. After receiving neoadjuvant chemotherapy and surgery, the tumor necrosis percentage was 91%. There are articles which support that a percentage of high tumor necrosis is associated with immediate levels above 1000 mcM, and that these in turn are correlated with a greater progression-free survival. In the above case, the results suggest that if the dose had been limited according to the cited recommendations, the tumor necrosis percentage would not exceed 90%.
Conclusions: The idiosyncrasy of each patient and the pharmacokinetics of each drug should be the factors that determine drug adjustment.
Key Words: Patient safety, osteosarcoma, body surface area.
____
INTRODUCTION
Drugs are mostly dosed according to the patient’s weight, but there are exceptions. One is the case of cytostatics, which are usually dosed depending on body surface area (BSA)1.
In oncology practice it is usual to dose drugs to a BSA of 2 m2 in those patients with a BSA than this. This limitation is performed to avoid exposing patients to such high doses that they could produce more adverse effects related with dose. This accepted belief is not really documented in any publication, however, articles do exist that support that the use of the dose based on the actual weight of the patient, despite exceeding BSA=2 m2, it does not increase adverse effects1.
Osteosarcoma is a tumor that occurs primarily during the growing age and is more common in males2, and it is located mainly in the metaphysis of long bones. The survival of patients with osteosarcoma has improved since the start of treatment with high-dose methotrexate (HDMTX)3.
The efficacy of treatment with HDMTX, expressed on the basis of progression-free survival data, has been linked to a concentration at the end of infusion above 1000 mcM and an area under the curve (AUC) greater than 4000 mg*hr/L, while reducing the infusion time of the drug is recommended in the case of not reaching this maximum concentration4. Current figures place the overall survival of localized disease at 62% and 7% for those with disseminated disease (in a follow-up period of 123 months). In a subgroup analysis for patients with localized disease and degree of tumor necrosis >90% after neoadjuvant treatment, progression-free survival was 70%, whereas for those with a degree of tumor necrosis < 90% it was 44%5.
Regarding safety, the toxicity profile of methotrexate (MTX) is associated with adverse effects at hematological, renal, liver, gastrointestinal and neurological. Hematological toxicity and mucositis have been related to sustained plasma levels of MTX rather than the peak reached6.
DESCRIPTION OF CASE
A 36 year old male, was diagnosed in August 2013 with high grade osteosarcoma in the left distal femur, anatomical pathology refers to bone infiltration due to high grade sarcoma.
Anthropological data corresponded to 115 kg and 1,92 m in height (according to DuBois) that correspond to a 2,44 m2 BSA. Creatinine clearance before beginning the protocol was 187 ml/min (according to Cockcroft-Gault).
The patient began neoadjuvant chemotherapy on 9th November with MTX 12 g/m2, adriamycin 75 mg/m2 (ADM) and cisplatin 90 mg/m2 (CDDP). CDDP was modified by ifosfamide 3000 mg/m2 (IFO) during the protocol as the patient had a sensorineural hearing loss. Finally, the scheme that followed was MTX (day +1), CDDP-ADM (+8), MTX (+32), MTX (+37), MTX (+44), IFO-ADM (+58), MTX (+78).
After these 7 cycles, surgery (+102) is performed.
Finally, he received adjuvant chemotherapy with MTX (+129), IFO-ADM (+140), MTX (+164), MTX (+172), IFO-ADM (+183) MTX (+204), MTX (+212), IFO-ADM (+223).
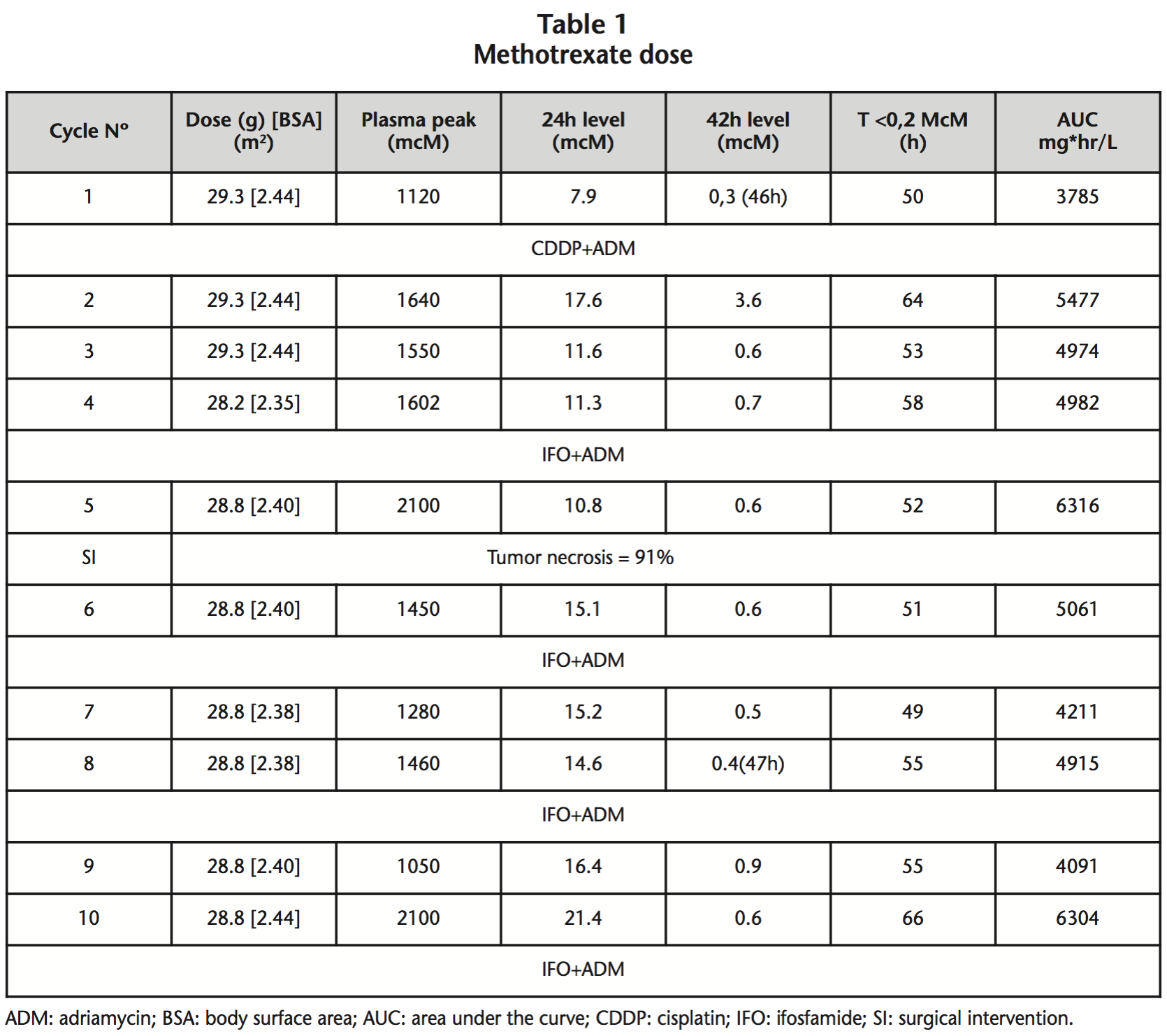
MTX was adjusted to 12 g/m2 (without dose limitation for BSA or total dose) in a 4 hour infusion. MTX immediate plasma levels at 24 h, 42 h, the time until MTX plasma concentrations were <0.2 mcM and the obtained AUC are reported in table 1.
The Bayesian method implemented in Abbottbase Pharmacokinetics Systems (PKS) to predict plasma MTX concentration. MTX levels are considered at risk of toxicity when they are above, the following levels:
• [MTX]24h > 5 mcM
• [MTX]42h ≥ 1 mcM
In these situations, rescue measures are intensified, such as the hyper-hydration (increase from 3 L/m2 to 4,5 L/m2), folinic acid rescues (increase rescue from 15 mg/m2 to 500 mg/m2) and urinary alkalinization. If the levels are too high, special measures are resorted to such as the use of activated charcoal, cholestyramine, carboxypeptidase and/or renal replacement techniques.
DISCUSSION
MTX is a drug from which it is possible to obtain plasma levels. This fact allows us to perform dose adjustment besides that carried out by the BSA1, and thus to know what exposure the patient is undergoing, therefore adjusting the dose of subsequent cycles in cases in which they have not reached the desired levels7. Another feature of MTX is that rescues can be adjusted, with folinic acid, hydration and urinary alkalinization, managing to reverse intoxication in cases in which it was necessary.
After receiving the full treatment, the result of the anatomical pathology was: 8 cm high-grade osteosarcoma, limited to bone, tumor necrosis percentage of 91%, and resection margins free of neoplasia. There are articles4,5 hat support that the percentage of tumor necrosis is associated with the immediate levels achieved. In our case we can observe that these levels obtained were within the range that is related to efficacy (>1000 mcM), and which in turn, correlate with a tumor necrosis percentage >90%.
At 42 hours, the levels obtained were not toxic, except the second cycle, in which they were slightly toxic, obtaining plasma levels of <0,2 mcM at 64 hours. In the first cycle reversible elevated hepatotoxicity of liver enzymes was shown, something relatively frequent in the use of MTX; at no time was any dose adjustment made.
Moreover, Fleming et al.8 a studied that what is really important for dose adjustment of MTX is not the weight, but the renal function, as approximately 90% of the drug is eliminated this way.
All these reasons lead us to question what is collected in most osteosarcoma protocols, in which it is recommended to limit the total dose to 20 g or adjust the dose to 2 m2 of maximum BSA for those patients with a BSA above this5,9.
In the aforementioned case, the data suggest that if the dose had been limited to 20 g or BSA of 2 m2 so as to try to avoid toxicity, a percentage of tumor necrosis above 90% would possibly have not been reached, as the outcome of the pathology was slightly higher with 91% tumor necrosis.
CONCLUSIONS
It would be interesting to perform studies in which patients in whom the dose is limited to 2 m2 and in which they are dosed according to real-BSA are compared and check whether there are significant differences regarding effectiveness. On the other hand, it would be necessary to perform pharmacokinetic studies of drugs prior to recommending this limitation of the dose.
Each patient’s idiosyncrasy and the pharmacokinetics of each drug should determine the dose adjustment.
Conflict of interest: The authors declare no conflicts of interest.
BIBLIOGRAPHY
1. Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012 May 1;30(13):1553-61.
2. Registro Nacional de Tumores Infantiles (RNTI-SEHOP) [updated 19 Feb 2015]. Available from: http://www. uv.es/rnti/informes.html.
3. Choeyprasert W, Pakakasama S, Sirachainan N, Songdej D, Chuansumrit A, Anurathapan U, Hongeng S, Nartthanarung A. Comparative outcome of Thai pediatric osteosarcoma treated with two protocols: the role of high-dose methotrexate (HDMTX) in a single institute experience. Asian Pac J Cancer Prev. 2014;15 (22):9823-9.
4. Aquerreta L, Aldaz A, Giráldez J, Sierrasesúmaga L. Methotrexate pharmacokinetics and survival in osteosarcoma. Pediatr Blood Cancer. 2004 Jan;42(1):52-8.
5. Protocolo de tratamiento del sarcoma osteogénico con enfermedad localizada en niños y adolescentes. Sociedad Española de Hematología y Oncológia Pediátricas. Mayo 2010.
6. Holmboe L, Andersen AM, Mørkrid L, Slørdal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012 Jan;73(1):106-14.
7. Aquerreta L, Aldaz A, Giráldez J, Sierrasesúmaga L. Pharmacodynamics of high-dose of methotrexate in pediatric patients. Ann Pharmacother. 2012 Sept;36 (9):1024-7.
8. Fleming RA, Eldridge RM, Johnson CE, Stewart CF. Disposition of high-dose methotrexate in an obese cancer patient. Cancer. 1991 Sep 15;68(6):1247-50.
9. Protocolo de estudio post-autorización observacional. Estudio prospectivo observacional de la expresión de ABCB1/P-glicoproteína como factor para la estratificación biológica del osteosarcoma no metastático de las extremidades. Grupo Español de Investigación en Sarcomas (GEIS) Versión 3.1 del 14 de noviembre de 2013.
____
Download PDF: High-doses of methotrexate in osteosarcoma. Does it adjust to a real body surface area?
