Rev. OFIL 2018, 28;1:29-32
Fecha de recepción: 12/10/2017 – Fecha de aceptación: 10/12/2017
García-Lagunar MH, Martínez-Penella M, Chica-Marchal AM, Viney AC, Muñoz-García I, Mira-Sirvent MC
Departamento de Farmacia. Hospital General Universitario Santa Lucía (HGUSL). Cartagena (España)
____
Correspondencia:
María Henar García Lagunar
Hospital General Universitario Santa Lucía
(Departamento de Farmacia Hospitalaria)
C/Mezquita s/n, Paraje Los Arcos
30202 Cartagena (Murcia)
Correo electrónico: henargl@gmail.com
____
Abstract
Objectives: To describe the actions carried out in a Spanish tertiary hospital to adapt the handling of hazardous drugs to the recommendations of the National Institute for Occupational Safety and Health, and the impact these changes have had on the Hospital Pharmacy´s workload.
Methods: A retrospective observational study was carried out to identify the hazardous drugs included in the hospital´s pharmacotherapeutic guide and to classify these according to the recommendations established for the handling of hazardous drugs, including storage, packaging, preparation and dispensation.
Results: One hundred and eighty-three hazardous drugs were found to be included in the hospital´s pharmacotherapeutic guide and thus were classified into 6 categories based on the established recommendations. Furthermore, the repackaging of drugs and the compounding of medications included in the list of hazardous drugs were reviewed, identifying 5 drugs that required manual repackaging and 5 compounded medications that required preparation in a Biological Safety Cabinet. Regarding the drug administration, the Hospital Pharmacy also provided a poster with recommendations for the handling of hazardous drugs for the different hospital departments.
Conclusions: The actions carried out by the Hospital Pharmacy suppose a decrease in the risk of occupational exposure in nursing staff, minimizing the handling of hazardous drugs with a consequent increase in safety.
Key Words: Hazardous drugs, occupational exposure, safety, handling drugs.
Introduction
Early concerns about occupational exposure to antineoplastic drugs first appeared in the 1970s1. Antineoplastic drugs are not the only drugs that can be considered hazardous, other drugs can be considered hazardous because of their potential to cause irreversible effects.
The National Institute for Occupational Safety and Health (NIOSH) published a list of major hazardous drugs in September 2004 (Preventing Occupational Exposures to Antineoplastic and other Hazardous Drugs in Healthcare Settings)1. This list was updated in 20102, 20123, 20144 and 20165. The current list (NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings)5 gives standard precautions for when handling hazardous drugs, but safe-handling precautions can vary with the activity and the formulation of the drug. The current NIOSH approach involves three groups of drugs:
- Group 1: antineoplastic drugs.
- Group 2: non-antineoplastic drugs that meet one or more of the NIOSH criteria for a hazardous drug: carcinogenicity, teratogenicity or other developmental toxicities, reproductive toxicity, organ toxicity at low-doses, genotoxicity or structure and toxicity profiles of new drugs that mimic existing drugs determined hazardous by the above criteria.
- Group 3: drugs that primarily pose a reproductive risk to men and women who are actively trying to conceive and women who are pregnant or breast feeding, but do not pose a risk to the rest of the staff.
In Spain, the “Instituto Nacional de Seguridad e Higiene del Trabajo” (INSHT) published a Technical Note on Prevention NTP7406 of Occupational Exposure to antineoplastic drugs in health care settings, which listed the major hazardous drugs and indicated specific recommendations to promote healthcare worker safety in all phases of the drug management cycle including the acquisition, storage, preparation, repackaging, dispensing, administration and identification of drugs. Subsequently, in September 2016, the INSHT published a technical document adapting the NIOSH list to the drugs used in Spain, giving recommendations on the preparation and the administration, taking into account the presentations and pharmaceutical forms used in Spanish hospitals. This document is pioneer in the world, being Spain the first country to adapt the NIOSH list to its own working environment7.
The aim of the present work is to describe the actions carried out in a Spanish tertiary hospital to adapt the handling of hazardous drugs to the NIOSH and INSHT recommendations, and how these changes have impacted on the Hospital Pharmacy’s workload.
Material and methods
A retrospective observational study of the measures put into place by the Pharmacy service and the Occupational Hazard Prevention service to adapt to the new recommendations and requirements when handling hazardous drugs.
Firstly, we identified the hazardous drugs included in the hospital’s pharmacotherapeutic guide and classified them according to their handling recommendations and the necessary actions that needed to be taken in terms of their storage, repackaging, preparation and dispensation.
The pharmacotherapeutic guide and the guidelines for the administration of drugs via a nasogastric tube were modified to adapt them to the changes made. Finally, all the actions carried out were communicated to the Hospital’s Management Team and the information about drug administration was distributed in posters and newsletters to the nursing staff.
Results
Of the 321 pharmaceutical forms included in the INSHT list, 134 were excluded because they were not included in the hospital’s pharmacotherapeutic guide and 4 drugs were withdrawn from the guide because they were considered hazardous and had low level of consumption in the hospital. The 183 drugs included were classified as:
- 101 drugs from group 1.
- 44 drugs from group 2.
- 38 drugs from group 3.
According to the route of administration they were classified in:
- 81 parenteral drugs (vials, ampoules, pre-filled syringes).
- 98 oral drugs (tablets, capsules, lozenges, oral solutions).
- 2 vaginal drugs (tablets).
- 1 ophthalmic drug (ointment).
- 1 topical drug (ointment).
The Pharmacy Service classified the drugs into 6 groups according to the route of administration and the hazard criteria to simplify the actions required:
- 63 parenteral antineoplastics drugs that are prepared in a class IIb Biological Safety Cabinet (BSC) following all the recommendations of preparation (double gloves, a protective gown, eye protection and FFP3 filtering facepiece respirators).
- 38 oral antineoplastic (group 1) drugs for which the guidelines for the administration of drugs via a nasogastric tube and the pharmacotherapeutic guide have been modified to indicate that they should not be split or crushed, and that if these actions are necessary, nursing staff should consult first with the Hospital Pharmacy service to assess whether the treatment is temporally stopped, the drug is administered via another route or splitting is done by the Pharmacy service in a class I BSC.
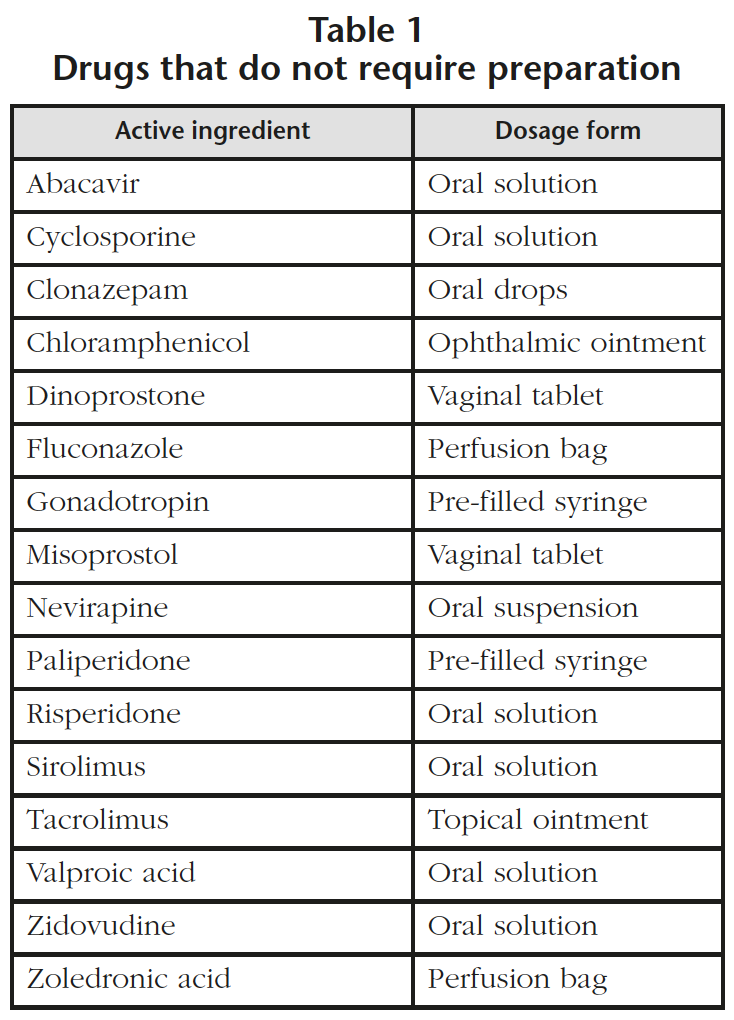
- 16 drugs that do not require any further manipulation in regards to compounding, although they do require special recommendations for administration (Table 1). When it is necessary, unit doses of oral solutions are prepared in a BSC with double gloves, a protective gown and FFP3 respirators.
- 49 oral drugs from groups 2 and 3 for which the pharmacotherapeutic guide and the guidelines for the administration of drugs via a nasogastric tube have been modified to state that they should not be split or crushed, and that if these actions are necessary, nursing staff should consult first with the Hospital Pharmacy service to assess whether the treatment is temporally stopped, the drug is administered via another route or splitting is done in a class I BSC. In this group is acenocoumarol for which the dose of 4 mg has been withdrawn from the pharmacotherapeutic guide to avoid splitting, using 1 mg tablet form for all dose requirements.
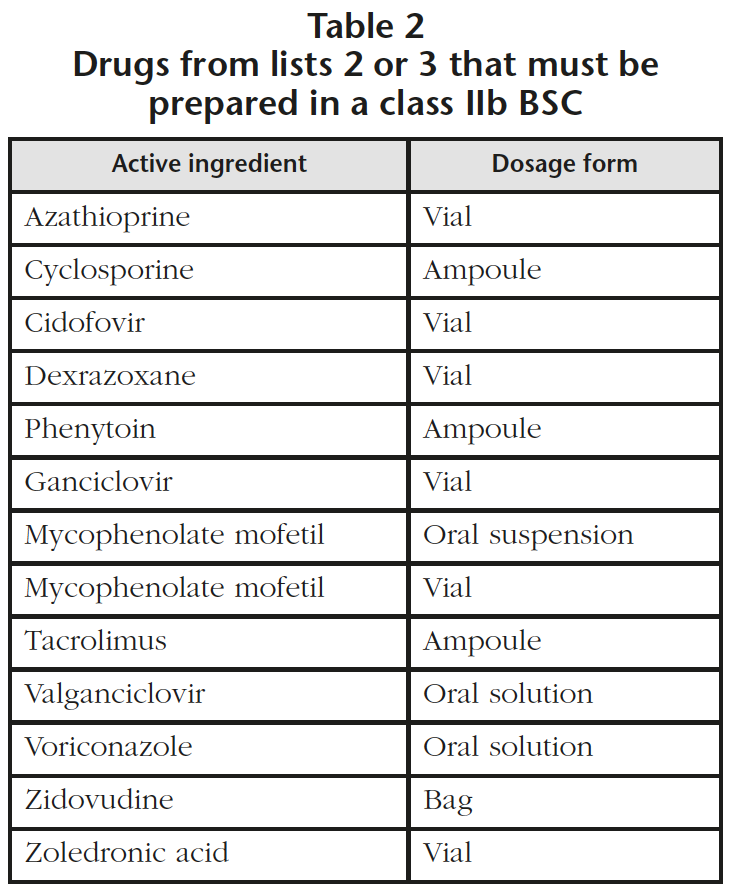
- 13 drugs from groups 2 and 3 (oral suspension marketed as powder and parenteral lyophilized powder) that must be reconstituted in a class IIb BSC (Table 2). It was detected that 6 drugs that required preparation in a class IIb BSC had not been prepared previously using this method and it has been estimated that this supposes an increase of 350 units per year. In the specific case of zoledronic acid, the perfusion bag (Table 1) is used when a 4 mg dose is needed and other doses are prepared with vials of zoledronic acid (Table 2) in a class IIb BSC.
- 4 parenteral drugs from group 3 for which protection is needed only if the handler is at reproductive risk. As a general rule, these drugs should not be handled by personnel at reproductive risk, but in exceptional cases when these drugs must be prepared, handlers will be protected with double gloves, gowns, eye protection and FFP3 respirators. These measures were agreed upon by the Pharmacy service and the Occupational Hazard Prevention service. With regard to oxytocin, it is only hazardous for pregnant women in the 3rd trimester. At this stage the midwives are already on maternity leave, so the risk to the handlers is reduced or even disappeared.
The review of hazardous drugs led to some changes in the acquisition of certain drugs. In addition to 4 drugs being removed from the pharmacotherapeutic guide, a modification in the acquisition process of one drug was made (Borea® powder was changed to Megefren® tablets) to avoid dissolving the powder and disposing of the unitary format. All these modifications were indicated in the phamacotherapeutic guide.
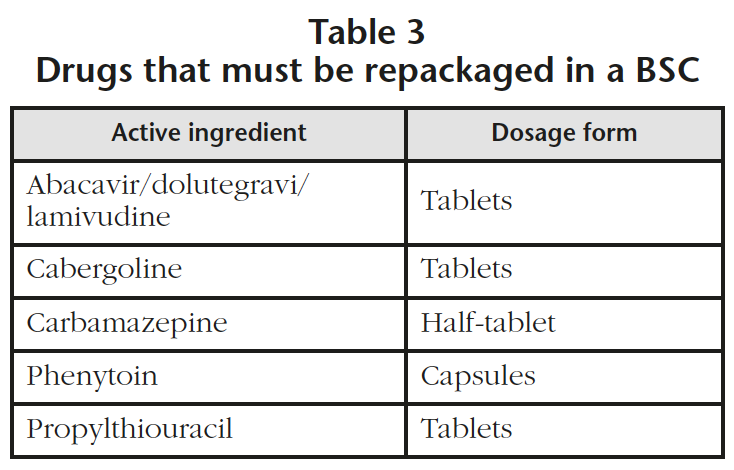
The areas dedicated to the repackaging of drugs and the compounding of non-sterile medications, were identified as high risk areas. A review of the repackaging process was performed in the hospital identifying 5 drugs that required to be manually repackaged in a class I BSC to avoid using the automatic repackaging machines (Table 3). This modification will suppose an increase of 2,000 units of manual repackaging per year. The repackaging of oral drugs from list 1 was already being done with a manual repackaging in a class IIb BSC.
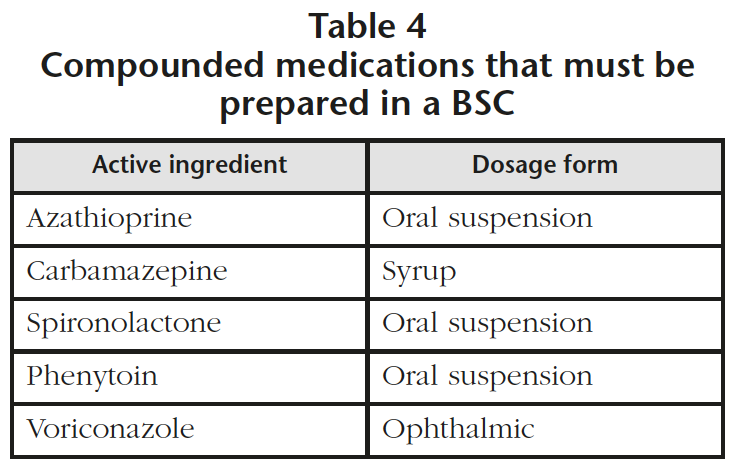
All the Standard Operating Procedures (SOPs) of the Pharmacotechnology and Intravenous preparations section involving hazardous drugs from lists 2 or 3 were reviewed and modified if necessary, detecting 5 compounded medications that required their preparation in a BSC (Table 4). Detailed instructions were included in the SOPs to ensure a safe preparation process of these drugs. For the molecules included in list 1, there was no detection of any new activities to be conducted because these drugs were already being prepared in the BSC.
The Pharmacy service together with the Occupational Hazard Prevention service classified all drugs included in the INSHT list into two categories according to the recommendations of administration: drugs that should be administered with single gloves (tablets and capsules) and drugs that should be administered with double gloves, gowns, eye protection (goggles) and respiratory protection (FFP3 respirators) in case of the respective risks of splashing or inhalation.
Information posters and newsletters with all the recommendations concerning the administration of hazardous drugs were made for the different hospital departments. Identification stickers were acquired for the drugs from lists 2 and 3 because stickers with the message “CYTOSTATIC DRUG” (for drugs from group 1) were already available in the hospital.
Discussion
The recommendations indicated from NIOSH and INSHT have triggered a major change in the way medication is handled5,7, both by the number of new drugs considered hazardous and by the changes in the management of their administration, therefore the Hospital Pharmacy services should actively collaborate in establishing, implementing and complying with these recommendations to contribute to minimize the risks of occupational exposure to hazardous drugs, as well as the regular updating of these recommendations to ensure a continuous improvement in the level of safety.
The new system that has been implemented in our hospital ensures a correct handling of hazardous drugs, simplifying the necessary measures for their preparation and administration. It should be noted that these actions have meant a reevaluation of the SOPs of the Pharmacy service including the processes of repackaging and compounding to minimize the risks of occupational exposure during the handling of these drugs.
One effective method that can be used in the preparation of antineoplastic drugs and other hazardous drugs is the use of Closed System drug Transfer Devices to transfer the drug mechanically, prohibiting the transfer of environmental contaminants into the system and the escape of active ingredients or vapor concentrations outside the system, thus ensuring that the preparation process is safe for the healthcare worker8.
These safety measures have already been carried out in many other hospitals, causing significant changes in the handling of drugs and requiring a review of the SOPs of the different Hospital Pharmacy services9,10. Each institution must evaluate the drugs used in its own center and the risks to its healthcare workers, adopting the necessary measures in each case depending on the initial situation of the center, with the requirement of more or fewer actions in order to ensure a safe use of hazardous drugs in each hospital.
One of the key aspects in the handling of hazardous drugs is the information and training program for all personnel revealing the potential risks and the way to work appropriately. One of the advantages of hospitals that have an electronic prescription program with an administration registration system, is that they can incorporate alerts on drugs with special recommendations including information about the handling and the administration of hazardous drugs.
Consequently, when a new drug is introduced into the pharmacotherapeutic guide, it must be checked to see whether it is classified as a hazardous drug or not, taking this information into account to select the appropriate pharmaceutical form (tablets or capsules better than envelopes, pre-filled syringes better than vials or ampoules) to avoid occupational exposure to the drug.
Finally, it is important to state the necessary actions when handling hazardous drugs in the hospital’s pharmacotherapeutic guide and in the guidelines for the administration of drugs via a nasogastric tube.
Conclusions
The actions taken suppose a decrease in the risk of occupational exposure in nursing staff, minimizing the handling of hazardous drugs with a consequent increase in safety.
On the other hand, these modifications have led to an increase in the Hospital Pharmacy’s workload regarding the updating of hospital guides, the increase in the number of compounded medications and the labelling work to identify the hazardous drugs.
Conflict of interests: The authors declare no conflict of interests.
Bibliography
- National Institute for Occupational Safety and Health (NIOSH). NIOSH alert: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Healthcare Settings [Internet] Cincinnati, September 2004. Available in: https://www.cdc.gov/niosh/docs/2004-165/.
- National Institute for Occupational Safety and Health (NIOSH). NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2010. [Internet] Cincinnati, September 2010. Available in: https://www. cdc.gov/niosh/docs/2010-167/pdfs/2010-167.pdf.
- National Institute for Occupational Safety and Health (NIOSH). NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2012. [Internet] Cincinnati, June 2012. Available in: https://www.cdc.gov/ niosh/docs/2012-150/pdfs/2012-150.pdf.
- National Institute for Occupational Safety and Health (NIOSH). NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2014. [Internet] Cincinnati, September 2014. Available in: https://www. cdc.gov/niosh/docs/2014-138/pdfs/2014-138.pdf.
- National Institute for Occupational Safety and Health (NIOSH). NIOSH list of antineoplastic and other hazardous drugs in healthcare settings 2016. [Internet] Cincinnati, September 2016. Available in: https://www. cdc.gov/niosh/topics/antineoplastic/pdf/hazardous-drugs-list_2016-161.pdf.
- Instituto Nacional de Seguridad e Higiene del Trabajo. NTP740: Exposición laboral a citostáticos en el ámbito sanitario. [Internet] Madrid, 2006. Available in: http:// www.insht.es/InshtWeb/Contenidos/Documentacion/FichasTecnicas/NTP/Ficheros/701a750/ntp_740.pdf.
- Instituto Nacional de Seguridad e Higiene del Trabajo. Medicamentos peligrosos: medidas de prevención para su preparación y administración. [Internet] Barcelona, September 2016. Available in: http://www. insht. es/InshtWeb/Contenidos/Documentacion/FICHAS%20DE%20PUBLICACIONES/EN%20CATALOGO/Higiene/2016%20medicamentos%20peligrosos/Medicamentos%20peligrosos.pdf.
- González-Haba Peña E, Manrique Rodríguez S, Herranz Alonso AM, Pérez Castán P, Moreno Gálvez M, Iglesias Peinado I, et al. Comparative study of preparation of hazardous drugs with different closed-system drug transfer devices by means of simulation with fluorescein. Farm Hosp. 2016;40(n06):496-503.
- García-Alcántara BG, Perelló-Palomar C, Moreno Centeno E, Modamio P, Mariño EL, Delgado Sánchez O. Impacto de las nuevas recomendaciones de manipulación de medicamentos peligrosos en un servicio de farmacia. Farm Hosp. 2017;41(2):257-269.
- Gaspar Carreño M, Achau Muñoz R, Torrico Martín F, Agún González JJ, Sánchez Santos JC, Cercos Lletí AC, Ramos Orozco P. Desarrollo de un procedimiento para el manejo seguro de medicamentos peligrosos. Farm Hosp. 2017;41(2):222-256.
____
Download PDF: Implementation of new recommendations for handling hazardous drugs
