Oterino-Moreira I1, Sanz Márquez S1, Zhan Zhou E1, Martínez Simón JJ1, Morales Catalán MC2, Lorenzo Martínez S2, Pérez Encinas M1
1 Pharmacy Department. 2 Quality and Management Department. Hospital Universitario Fundación Alcorcón. Alcorcón. Madrid (Spain)
Fecha de recepción: 03/08/2021 – Fecha de aceptación: 15/09/2021
Correspondencia: Iván Oterino Moreira – Hospital Universitario Fundación Alcorcón (Servicio de Farmacia) – C/Budapest, 1 – 28922 Alcorcón. Madrid (España)
ivan_mby@usal.es
____
SUMMARY
Background: At the beginning of the COVID-19 pandemic many drugs were used with an uncertain benefit/risk profile that needed to be evaluated. The goal of this study was to analyse the incidence of adverse drug reactions (ADRs) and describe the drugs used in COVID-19 hospitalised patients at the beginning of the COVID-19 pandemic through the minimum basic data set (MBDS).
Methods: Retrospective observational study that included hospitalised patients with COVID-19 at our centre between March and May 2020 who had ADRs coded in discharge/death medical reports according to the International Classification of Diseases (ICD-10). Those patients with ADRs ascribed to COVID therapy were selected and the causal relationship was evaluated using the Naranjo algorithm. Descriptive statistical analysis was used.
Results: We identified 141 ADRs in 110 cases of hospitalisation due to COVID-19 that entailed an incidence of 9.66% (141/1459), CI95% 8.25-11.29. From the ADRs analysed, 60.3% (85/141) were ascribed to COVID therapy. Lopinavir/ritonavir represented 38.8% (33/85) of ADRs, glucocorticoids 23.5% (20/85) and hydroxychloroquine 9.4% (8/85).
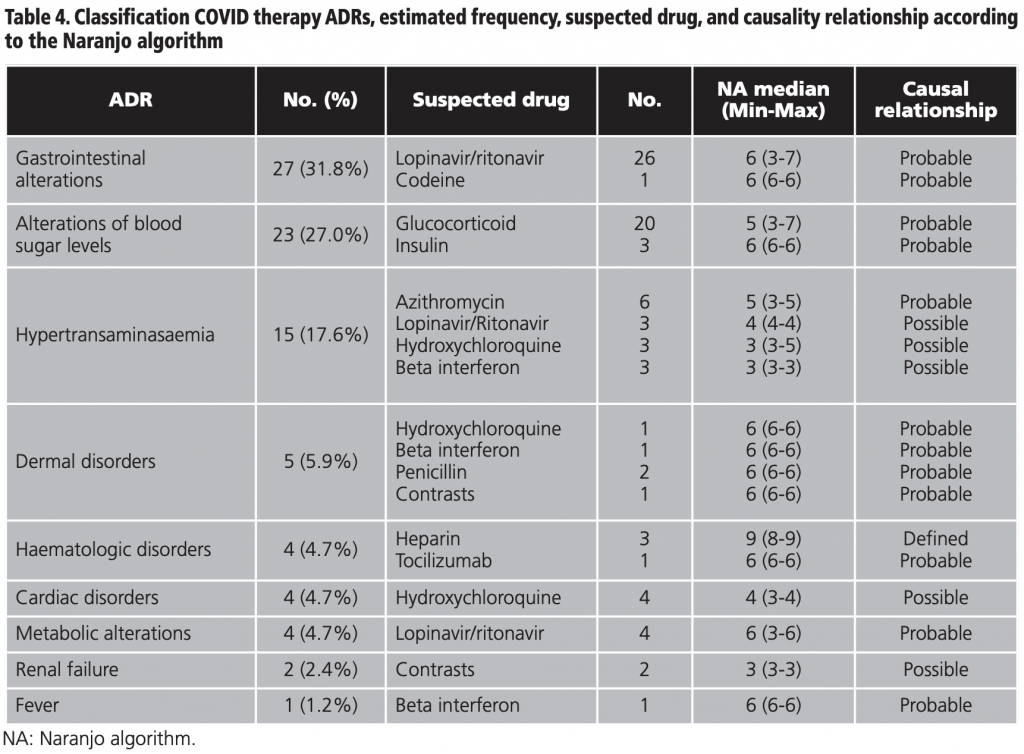
Out of the ADRs, 31.8% (27/85) were gastrointestinal disorders (probable lopinavir/ritonavir), 27.0% (23/85) blood glucose disorders (probable glucocorticoid) and 17.6% (15/85) hypertransaminasaemia (probable azithromycin, possible lopinavir/ritonavir, possible hydroxychloroquine, possible interferon).
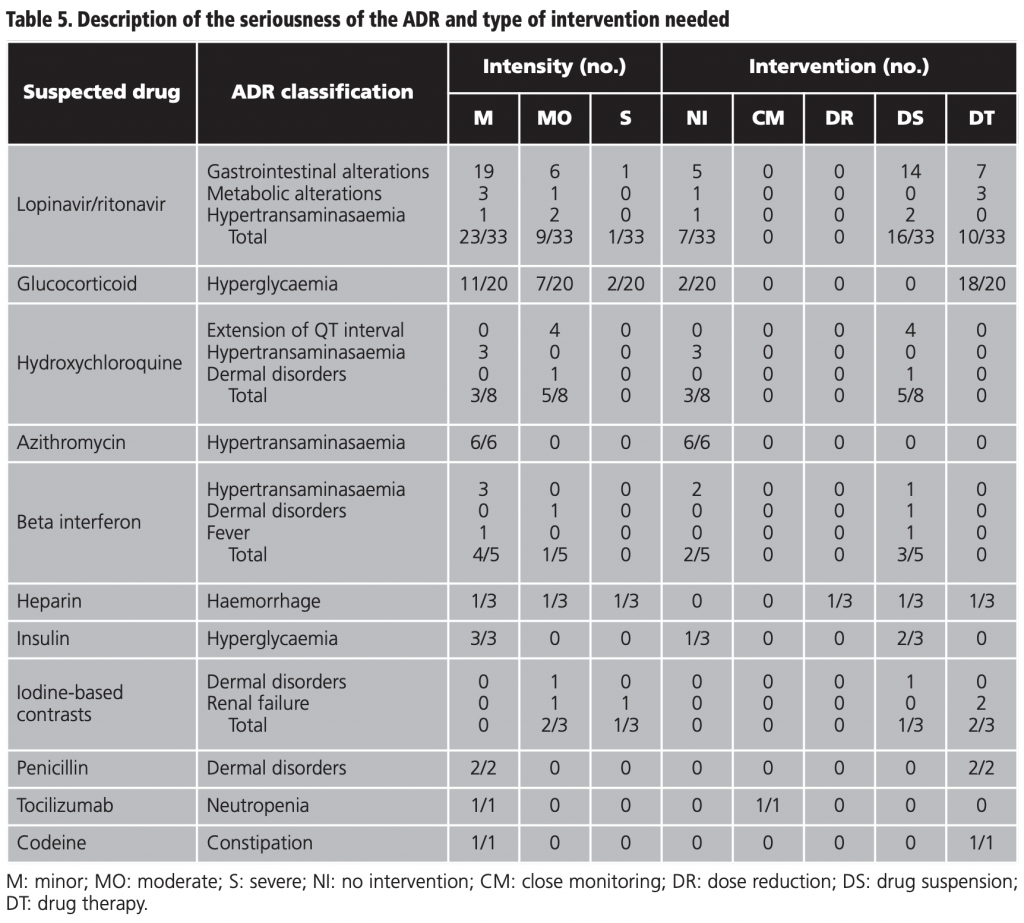
Regarding intensity, 64.7% (55/85) were mild cases, 29.4% (25/85) moderate and 5.9% (5/85) severe. The percentage of ADRs that did not require intervention were 24.7% (21/85), 32.9% (28/85) required pharmacological treatment, 40.0% (34/85) suspension of the drug, 1.2% (1/85) close monitoring and 1.2% (1/85) dose reduction.
Conclusions: The incidence of ADR in COVID population that required admission at the beginning of the pandemic seems to be higher than in the general population. The MBDS proves to be a useful tool to trace ADRs.
Key words: Minimum basic data set (MBDS), adverse drug reaction (ADR), COVID-19 therapy, Naranjo algorithm, causality relationship.
Incidencia de reacciones adversas a medicamentos en pacientes hospitalizados por COVID-19 a través del conjunto mínimo básico de datos
RESUMEN
Introducción: La llegada de la pandemia de COVID-19 supuso la utilización de muchos fármacos con un perfil de riesgo/beneficio incierto que debe ser evaluado. El objetivo de este estudio fue analizar la incidencia de reacciones adversas a medicamentos (RAM) y describir los medicamentos utilizados en pacientes hospitalizados por COVID-19 al comienzo de la pandemia a través del conjunto mínimo básico de datos (CMBD).
Materiales y métodos: Estudio observacional retrospectivo que incluyó pacientes hospitalizados por COVID-19 en nuestro centro entre marzo y mayo de 2020 que presentaban RAM codificadas en los informes médicos de alta/exitus según la Clasificación Internacional de Enfermedades (CIE-10). Se seleccionaron los pacientes con RAM atribuidas a la terapia COVID-19 y se evaluó la relación causal mediante el algoritmo de Naranjo. Se realizó un análisis estadístico descriptivo.
Resultados: Identificamos 141 RAM en 110 casos de hospitalización por COVID-19 lo que supone una incidencia del 9,66% (141/1459), IC95% 8,25-11,29. De las RAM analizadas el 60,3% (85/141) se atribuyeron a la terapia COVID. Lopinavir/ritonavir representó el 38,8% (33/85) de las RAM, los glucocorticoides el 23,5% (20/85) y la hidroxicloroquina el 9,4% (8/85).
De todas las RAM, el 31,8% (27/85) fueron trastornos gastrointestinales (probable lopinavir /ritonavir), el 27,0% (23/85) trastornos de la glucemia (probable glucocorticoide) y el 17,6% (15/85) hipertransaminasemia (probable azitromicina, posible lopinavir /ritonavir, posible hidroxicloroquina, posible interferón).
En cuanto a la intensidad, el 64,7% (55/85) de las RAM fueron casos leves, el 29,4% (25/85) moderados y el 5,9% (5/85) graves. El porcentaje de RAM que no requirió intervención fue 24,7% (21/85), 32,9% (28/85) requirió tratamiento farmacológico, 40,0% (34/85) suspensión del fármaco, 1,2% (1/85) seguimiento estrecho y 1,2% (1/85) reducción de dosis.
Conclusiones: La incidencia de RAM en la población con COVID-19 que requirió ingreso al inicio de la pandemia parece ser mayor que en la población general. El CMBD demuestra ser una herramienta útil para rastrear RAM.
Palabras clave: Conjunto mínimo básico de datos (CMBD), reacción adversa a medicamento (RAM), terapia COVID-19, algoritmo de Naranjo, relación de causalidad.
____
INTRODUCTION
After the identification in China of a new type of virus from the family Coronaviridae causative of pneumonia in humans, the World Health Organization (WHO) declared a global pandemic on the 11th of March 2020. As of 15 January 2020, more than 90 million cases were reported worldwide1. As of 15 February 2020, the WHO had already declared the COVID-19 infodemic, warning of the excess of information that was being spread2,3. The current lack of therapeutics and the emergency to find a therapy before the thousands of cases of severe pneumonia, contributed to the spreading of data that included sensational and distorted information about the drugs; which could have resulted in an inappropriate and, as such, dangerous use4.
In an attempt to minimise the potential risks resulting from this behaviour, scientific societies and regulatory agencies quickly reviewed any previous evidence available to ensure a secure and effective drug therapy against the emergent disease3,4. In this sense, the choice of potential treatment was based on biological plausibility because of the lack of solid clinical trials as backup.
The symptoms of COVID-19 patients differ from those that use these drugs within the approved indications; which may affect the profile of adverse effects5. Therefore, drugs were used with an uncertain benefit/risk profile that needs to be evaluated.
The measure of the risk linked to hospital care is a matter of utmost importance for the health system both in its health dimension as in the economic, legal, social and media dimensions6. Adverse drug reactions (ADRs) are considered to be one of the primary causes of morbidity, mortality and increase in costs7,8. It has been estimated that they cause 5-10% of hospital admissions and they are present in 10-20% of hospitalised patients, which increases their average stay.
In the Spanish national study of adverse events (ENEAS), from 2005, linked to hospitalisation, ADRs were the most frequent cause as they represented 37.4% out of the total of adverse events (AEs) detected. The authors concluded that the knowledge and sensitisation among professionals will help prevent what is easily preventable6,9.
Mena et al. described that 4 out of 10 patients who died due to COVID-19 in a Spanish hospital suffered an AE associated with healthcare; ADRs being the primary cause of AEs (23.8%). The authors highlight the need to carry out a close surveillance of possible ADRs which derive from drugs administered without a clear evidence of effectiveness against the infection and in relation to a disease with a still uncertain treatment10.
The reports issued by the Spanish Pharmacovigilance System (SEFV-H) regarding ADRs of treatments used to treat infection by SARS-CoV-2, and published by the Spanish Agency of Medicines and Medical Devices (AEMPS), analyse the suspected ADRs that health professionals or citizens reported through the spontaneous reporting system (SRS). However, in the reports of the SEFV-H, the causality relationship between the suspected drug and the reported ADR is not evaluated. Therefore, there is no certainty that the suspected drug caused the adverse effect5.
The minimum basic data set (MBDS) is the largest administrative database maintained in Spain with standardised clinical data of hospitalised patients, as well as the main source of information on treated morbidity. It contains, for each hospitalisation, information about the patient’s demographics, coding of the main diagnosis, up to 19 secondary diagnoses, and 22 procedures –according to the International Classification of Diseases, tenth revision (ICD-10–, as well as the reasons for discharge, severity, and costs, among others. While the main diagnosis refers to that which lead to hospital admission, secondary diagnoses are diseases that coexist at the time of admission, or which develop during hospital stay, and influence the duration or treatment. The secondary diagnoses include the ADRs, which are defined as the disorders and/or damages caused when the drugs are used appropriately11,12. Therefore, the systemic analysis of the MBDS can be used to calculate the impact of the ADRs in the hospital setting13.
The incidence of adverse drug reactions (ADRs) in COVID-19 patients has not been evaluated in depth yet, but the findings of the observational studies suggest a high frequency in this population14. Mena et al. assessed the adverse events related to healthcare in patients infected with SARS-CoV-2 who died in a Spanish hospital and discovered that 23.8% of the patients suffered ADRs, therefore becoming the primary cause of adverse events.
The objective of this study was to analyse the incidence of adverse drug reactions (ADRs) and to describe the drug involved in COVID-19 hospitalised patients at the beginning of the pandemic, based on the hospital discharge data provided by the MBDS.
MATERIALS AND METHODS
A retrospective observational study was designed to analyse the safety profile of the COVID-19 therapy available on the basis of the different treatment protocols that the regulatory agencies made accessible during the first wave of the pandemic.
The study included all the hospitalised patients in our centre between March and May 2020 diagnosed with COVID-19 who had an ADR coded in their discharge/death letter, according to the data from the MBDS hospital discharge registry. Subsequently, the ADRs ascribed to COVID-19 therapy were selected, whilst those caused by the drugs assigned to the treatment of other concomitant diseases were excluded. The suspected drug was identified on the basis of the initial product information, especially the data sheet. The causality relationship between the suspected drug and the ADR was evaluated with the Naranjo algorithm (NA).
The variables collected were those corresponding to the demographics, admission, discharge, diagnosis during the hospitalisation process, seriousness of the ADR (mild: discomfort that does not alter normal daily activities; moderate: enough discomfort to reduce or affect normal daily activities; severe: disability to execute normal daily activities), and handling of the ADR.
In the MBDS, the ICD-10 code selected in our study as the primary diagnosis was B97.2, which describes coronavirus as the cause of the disease and as secondary diagnosis we selected the codes T36-T50 which describe ADRs11,13,15,16.
A univariate descriptive statistical analysis was carried out using the statistical programme SPSS version 22 of all the clinical and analytic variables studied. These are presented in absolute and relative frequencies for qualitative variables, whereas quantitative variables are presented using the primary measures of central tendency and dispersion.
RESULTS
During the study period, 141 ADRs in 110 discharges with COVID-19 diagnosis were reported (105 patients). The total number of COVID-19 patients discharged during that same period was 1459; as such, the incidence of ADRs associated with the COVID-19 population that required hospital admission was estimated to be 9.66% (141/1459), CI95% 8.25-11.29.
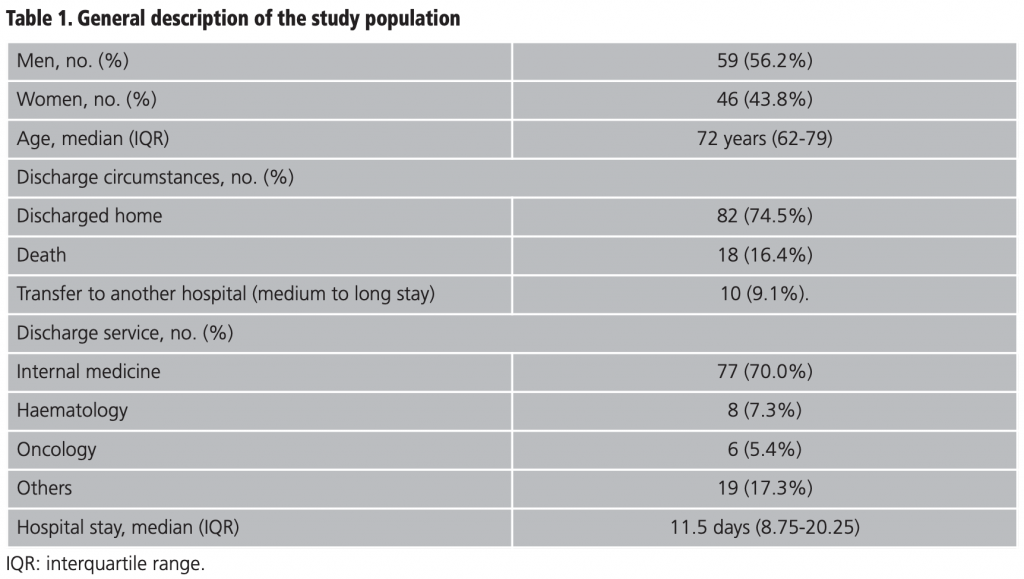
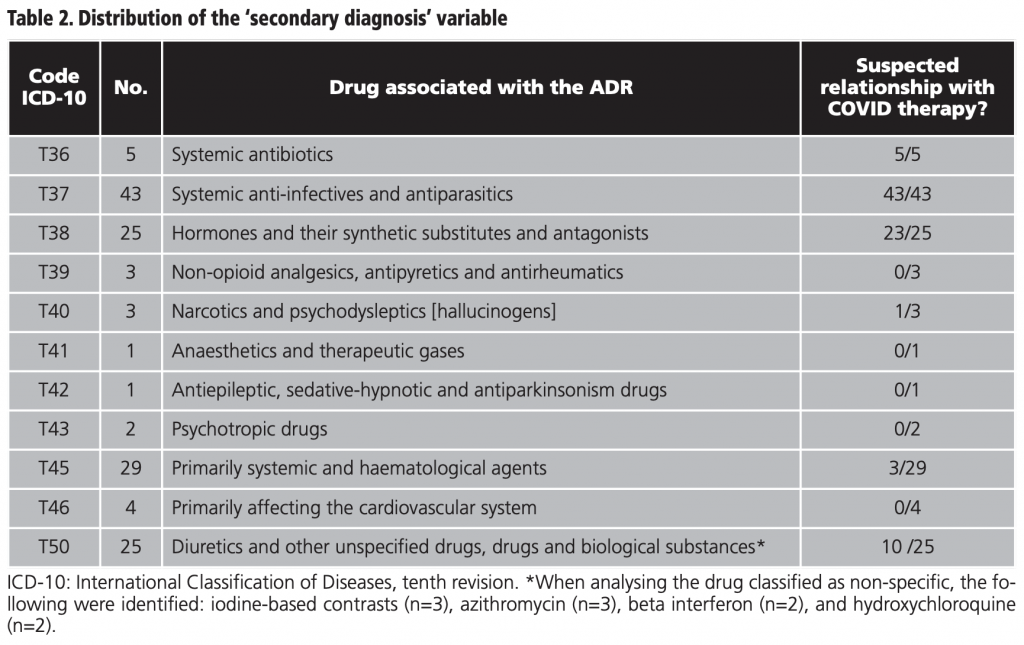
The general description of the study population is provided in detail in table 1. The male population represented 56.2% (59/105) and the median age was 72 years (IQR 62-79). The internal medicine service took on 70.0% (77/110) of the admissions and 74.5% (82/110) were discharged home. From those discharged, 16.4% (18/110) were due to death and 9.1% (10/110) were transferred to another hospital for medium to long stay. The median stay was 11.5 days (IQR 8.75-20.25). The median of different drugs was 16.5 (IQR 11-21) and the median duration of the ADR suspected drug was 4 days (IQR 7-11). The distribution of the secondary diagnosis variables (ADR) together with the official coding of the MBDS and its meaning15 are shown in table 2.
On the basis of table 2, out of all the ADRs reported (141), 60.3% (85/141) were ascribed to COVID therapy. The other patients included in the study (39/105) exhibited one or several ADRs during hospitalisation for COVID-19 but these were not necessarily caused by COVID therapy; as such, they were excluded in the subsequent analysis. According to this, the incidence of ADRs related to COVID therapy was 5.82% (85/1459), CI95% 4.74-7.15.
Lopinavir/ritonavir was associated with 38.8% (33/85) of the total of ADRs, followed by glucocorticoids with 23.5% (20/85) and hydroxychloroquine with 9.4% (8/85). To a lesser extent, beta interferon, heparin, insulin, contrasts, penicillin, tocilizumab, and codeine were also associated with ADRs (table 3).
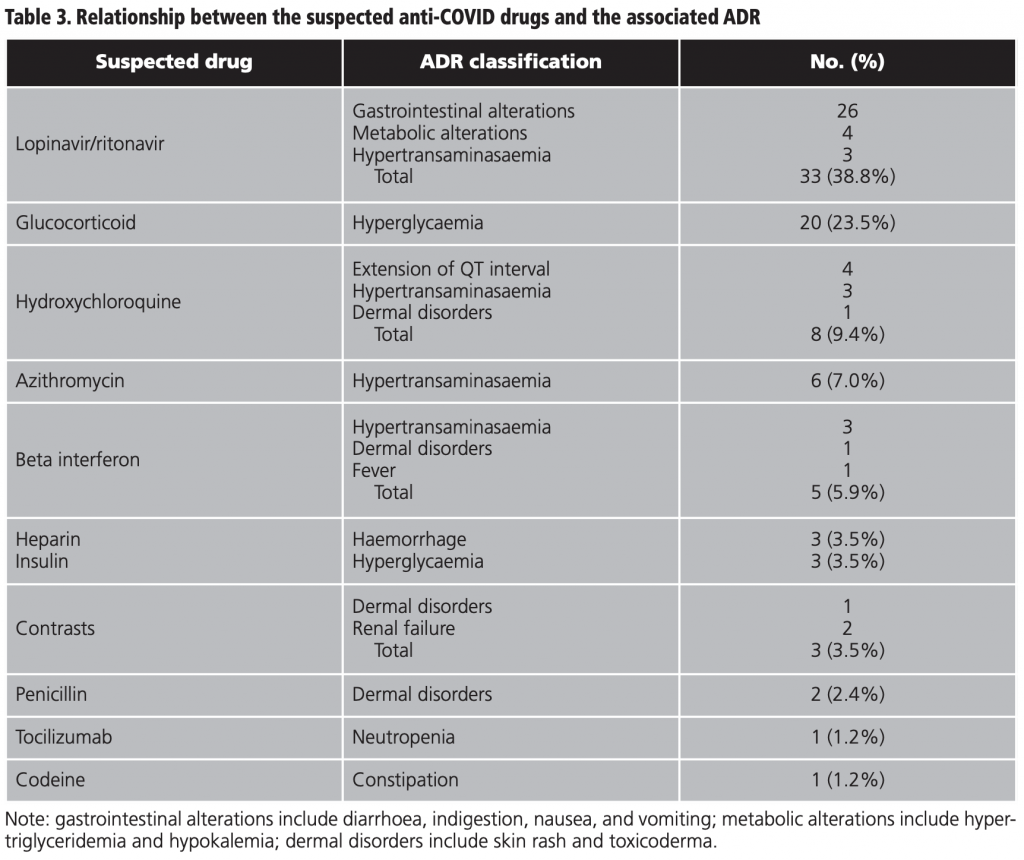
Table 4 shows the global classification of the ADRs detected together with the frequency calculated in the sample, the suspected drug, the median score in the Naranjo algorithm, and the causality relationship. Gastrointestinal alterations represented 31.8% (27/85) of all the ADRs analysed, followed by 27.0% (23/85) of alterations of blood sugar levels, and 17.6% (15/85) hypertransaminasaemia.
The intensity of the ADRs was mild in 64.7% (55/85) of the cases, moderate in 29.4% (25/85), and severe in 5.9% (5/85) of the cases. The percentage of ADRs which did not require intervention was 24.7% (21/85), 40% (34/85) required suspension of the drug, 32.9% (28/85) required drug therapy, 1.2% (1/85) close monitoring, and 1.2% (1/85) dose reduction. The detailed analysis by drug and ADR is shown in table 5.
DISCUSSION
We would like to highlight that the incidence rate of ADRs of any kind (9.66%) in COVID-19 hospitalised patients, as well as that associated exclusively with COVID-19 therapy (5.83%) in hospitalised patients, is higher than that reported in Spanish studies using a similar methodology (MBDS) for the general population: 0.89%13, 2.20%17, 2.15%18, and 5.5% for patients admitted to the internal medicine service of the hospitals of the Spanish National Health System19.
Few studies have reported incidence data in real life of ADRs associated with the COVID population. Most of the observational studies in the literature are based on the analysis of ADRs based on spontaneous reporting systems (SRS). The under-reporting, estimated to be 95-99%20-22, and the absence of a denominator related to the exposure to the drug, do not allow the SRS to estimate the incidence rate13,23. Only one study carried out in China between January-February 2020 using the China Hospital Pharmacovigilance System demonstrated an incidence of ADRs of 37.8%14 in the COVID population that took umifenovir, lopinavir/ritonavir, or chloroquine; however, it can hardly be compared due to the different therapies included in the analysis. These researchers identified gastrointestinal reactions, liver damage, anthema, and hyperlipidaemia with an incidence of 23.0%, 13.8%, 4.15%, and 1.38% respectively14.
In our study, we used the MBDS system to assess the safety issues of the COVID-19 therapy drugs for the first time. Several authors have defined the advantages of using the MBDS with pharmacovigilance purposes: it allows the identification of non-reported adverse events to the national pharmacovigilance centre –which can partly solve the insufficient reporting problem– and the study has a lower bias probability due to the effect in the measure of the results, owing to the research question, than studies based on the collection of primary data13,17,18,24,25; reflecting, in a better way, the ADRs for COVID-19 patients in the real world.
Our study demonstrated that ADRs in COVID-19 patients were mainly characterised by gastrointestinal alterations due to lopinavir/ritonavir, alterations of blood sugar levels due to corticosteroids, and hypertransaminasaemia due to azithromycin, lopinavir/ritonavir, hydroxychloroquine, and beta interferon.
As in the study of Sun et al., lopinavir/ritonavir was the drug that caused the highest number of ADRs14. These are ADRs that had already been described as frequent or very frequent in lopinavir/ritonavir clinical trials for its approved indications26 and our data correlates with the report of the SEFV-H regarding the suspected ADRs in treatments used for COVID-19 therapy, this reflects the gastrointestinal disorders as the most frequently reported as suspected ADRs due to lopinavir/ritonavir (47.0%)5.
A total of 45% of steroid-induced hyperglycaemias went from moderate to severe and in 90% of the cases a corrective insulin regimen was needed, which in turn was related to some cases of hypoglycaemia. The incidence of these ADRs –described in the data sheet of both drugs as frequent27,28– did not correlate with the report of suspected ADRs to drugs used for COVID-19 by the SEFV-H, in which the metabolic disorders represented only 7.14% of the ADRs for this group, probably due to under-reporting, since they are adverse effects widely known by health professionals and are easy to handle5. In accordance with our results, in a national study based on data from the MBDS, for patients admitted to the medicine service, steroids were the most frequently implicated group with ADRs (18.05% out of the total of ADRs) because of their effects on glucose metabolism19.
Cardiomyopathy, abnormal liver function, and anthema/ pruritus are described in the data sheet of hydroxychloroquine as associated with a rare, very rare, and frequent frequency respectively29. This data from clinical trials in its approved indications is not in line with the COVID population of our study nor with the report of the SEFV-H in which the haepatobiliary disorders have been the most frequently reported for the hydroxychloroquine therapy (36%), followed by the gastrointestinal (26%), cardiac (18%) and dermatological disorders (10%)5. In the study of Sun et al., the incidence of adverse effects due to chloroquine associated with COVID patients was 13.5% (9.4% in our sample for hydroxychloroquine), mainly exhibited as gastrointestinal alterations and liver damage (80.0% with possible causal relationship), also holding the third position in incidence after lopinavir/ritonavir and umifenovir14. Furthermore, in the study of Crescioli et al., that evaluated ADRs associated with COVID-19 therapy reported in an Italian hospital, 19 out of the 23 patients exhibited an extension of the QT interval, which was associated with the combination of hydroxychloroquine, azithromycin, lopinavir/ritonavir, and darunavir/cobicistat30. The aforementioned study is probably subject to a high selection bias towards the most severe ADRs.
The ADRs identified for beta interferon were also already described31. According to our results, the SEFV-H establishes haepatobiliary disorders as the most frequently reported (69%), although general disorders only represented 25.0%5. It is probable that general disorder ADRs (headache, fever, flu-like illness) are underestimated, both in the AEMPS report (under-reporting) and in our data from the MBDS (under-registered), as it is a commonly known ADR by clinicians, it can be controlled easily and it is unimportant in the patient’s global clinical progress.
We found three cases of induced haemorrhage due to heparin (1/3 required the transfusion of packed red cells). According to the data sheet of enoxaparin, haemorrhage is a frequent ADR32 and SEFV-H reports that blood disorders caused by heparins are the most frequently reported ADRs for this group (52%)5.
In relation to the iodine-based contrast media administered intravascularly, dermal toxicity is a well-known and described acute ADR in the literature33-35. The studies on the deterioration of the renal function are contradictory as they have become very contaminated by biases and combinations34. This last point is reflected in our results which cast a possible causality relationship (only 3 points in the NA, as the patients were also taking other nephrotoxic drugs, as such, reducing causality).
Neutropenia associated with the administration of tocilizumab is a frequent ADR that can be severe36. In the report of the SEFV-H, the haematologic disorders represented 33% of the reports of suspected ADRs by tocilizumab5. At our hospital, tocilizumab was saved for those patients in severe conditions and with a progressive increase of acute-phase reactants. Therefore, as opposed to the other drugs included in our study, it has not been administered to the entire sample, which could affect the low profile of detected ADRs.
Hypertransaminasaemia, caused by azithromycin, and dermal disorders, caused by amoxicillin/clavulanic, are described as rare or uncommon in authorised clinical trials37,38. Additionally, constipation due to codeine, of unknown frequency39, mild, and easy to handle is clearly under-registered in our sample.
Out of all the drugs analysed, as of the date of publication of this paper only one is allowed to be recommended with a high degree of evidence, that regarding the administration of glucocorticoids in hospitalised patients who need oxygen, azithromycin jointly with penicillin for suspected bacterial superinfection and anticoagulation with heparin5,40-44. The administration of tocilizumab in patients with high markers of systemic inflammation is a suggestion with a low evidential assurance45.
In the report of suspected ADRs due to COVID-19 therapy by the SEFV-H, 72% of the suspected ADRs were considered severe5. This has not been confirmed in our study in which we only found that 7.1% were severe. This discrepancy could be due to the mentioned information bias for the SRS, from which the SEFV-H feeds on, when identifying that, although also affected, the under-reporting is lower in more severe and rare adverse effects22, which tends to underestimate the milder and more frequent ADRs.
We do not have information on remdesivir given that it was not available during our study period. Anakinra, sarilumab, siltuximab, ruxolitinib, or baricitinib are drugs that have been used for COVID-19 but, currently, their use is only recommended in randomised clinical trials that allow us to create evidence43.
According to the literature, a high percentage of ADRs (57.20% to 62.3%) detected by the retrospective analysis of the MBDS are preventable17,18. Our study identifies potential unknown risks or risks with changes in the way already identified adverse effects appear, that allow us to take the appropriate preventative actions to encourage the safe use of drugs. It also establishes a methodological basis for complementary pharmacovigilance studies from those deriving from declarative registers that will be useful for notifying ADRs to the national surveillance system that, if not, would not be reported. Nevertheless, certain limitations and biases must be mentioned.
Firstly, the results included in the current findings may underestimate the incidence of ADRs due to the lack of control in the register of adverse events (under-register)24. It seems there is a tendency to register the more severe and marked adverse events to the detriment of those that are more mild, frequent, and easy to handle13. Another limitation of these types of studies is the possibility that coding errors are made, sometimes attributable to the variability in the codes, with reported error rates of >22%13.
The incidence of ADRs in the COVID population suggests that it is higher than the average and, although most went from mild-to-moderate, more than 75% required medical intervention. The review of the MBDS is a useful and easy to access method to identify a great number of ADRs caused by COVID-19 therapy and provides information about the primary drugs involved, which can be used to implement preventative strategies.
Conflict of interests: The authors declare that they do not present a conflict of interest.
BIBLIOGRAPHY
1. Center for the Coordination of Health Alerts and Emergencies, editor. [Scientific-technical information: Coronavirus disease, COVID-19]. [Internet]. Madrid: Ministry of Health; 2021 Jan. Available from: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos.htm.
2. Eysenbach G. How to Fight an Infodemic: The Four Pillars of Infodemic Management. J Med Internet Res. 2020 Jun;22(6):e21820. PubMed; PMID 32589589.
3. Tangcharoensathien V, Calleja N, Nguyen T, Purnat T, D’Agostino M, Garcia-Saiso S, et al. Framework for Managing the COVID-19 Infodemic: Methods and Results of an Online, Crowdsourced WHO Technical Consultation. J Med Internet Res. 2020 Jun;22(6):e19659. PubMed; PMID 32558655.
4. Tuccori M, Convertino I, Ferraro S, Cappello E, Valdiserra G, Focosi D, et al. The Impact of the COVID-19 «Infodemic» on Drug-Utilization Behaviors: Implications for Pharmacovigilance. Drug Saf. 2020 Aug;43(8):699-709. PubMed; PMID 32572842.
5. Working Group to follow up suspected ADR cases reported in treatments for SARS-CoV-2 infection. [Suspected adverse reactions reported with treatments used in COVID-19. Report No. 9: Data from March 2020 to January 2021]. [Internet]. Madrid: Ministry of Health: AEMPS; 2020 Dic. Available from: https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid%E2%80%9119/.
6. Aranaz Andrés JM, editor. [National Study on Adverse Effects linked to Hospitalization: ENEAS 2005]. Madrid: Ministry of Health; 2006 Feb. Available from: https://www.seguridaddelpaciente.es/es/proyectos/financiacion-estudios/e-epidemiologicos/2005/.
7. Belhekar MN, Taur SR, Munshi RP. A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014 Feb;46(1):117-120. PubMed; PMID 24550597.
8. Montané E, Santesmases J. Adverse drug reactions. Med Clin (Barc). 2020 Mar;154(5):178-184. PubMed; PMID 31771857.
9. Aranaz-Andrés JM, Aibar-Remón C, Vitaller-Burillo J, Requena-Puche J, Terol-García E, Kelley E, et al. Impact and preventability of adverse events in Spanish public hospitals: results of the Spanish National Study of Adverse Events (JANAS). International Journal for Quality in Health Care. 2009 Dec ;21(6):408-414. PubMed; PMID 19841027.
10. Mena G, Montané E, Rodríguez M, Beroiz P, López-Núñez JJ, Ballester M. Patient characterization and adverse health care-related events in SARS-CoV-2 infected patients who died in a tertiary hospital. Med Clin (Barc). 2020 Dec; S0025-7753(20)30818-6. PubMed; PMID: 33358536.
11. [Royal Decree 69/2015 of February 6, which regulates the Register of Specialized Health Care Activity]. Madrid: BOE, no. 35, (Feb 10, 2015). Available from: https://www.boe.es/.
12. [Hospitalization Report-Discharge Registry: 2014 Summary Report]. [Internet]. Madrid: Ministry of Health; 2016. Available from: www.msssi.gob.es/estadEstudios/estadisticas/cmbdhome.htm.
13. Salmerón-García A, Cabeza Barrera J, Vergara Pavón MJ, Román Márquez E, Cortés de Miguel S, Vallejo-Rodríguez I, et al. Detection of adverse drug reactions through the minimum basic data set. Pharm World Sci 2010 Jun; 32(3):322-328. PubMed; PMID 20213432.
14. Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, et al. Incidence of Adverse Drug Reactions in COVID-19 Patients in China: An Active Monitoring Study by Hospital Pharmacovigilance System. Clin Pharmacol Ther. 2020 Oct;108(4):791-797. Pub-Med; PMID 32324898.
15. Pastor Sanmillán MD, editor. [Coding Manual: ICD-10-ES Diagnostics 3rd ed]. Madrid: Ministry of Health; 2019 Dic. Available from https://eciemaps. mscbs.gob.es/ecieMaps/documentation/documentation.html.
16. [eCIE10ES: Electronic edition CIE10ES Diagnostics. 3rd ed]. [Internet]. Ministry of Health [updated Mar 2020; access Jan 2021]. Available from: https:// eciemaps.mscbs.gob.es/ecieMaps/browser/index_10_mc.html.
17. Sánchez Muñoz LA, Castiella Herrero J, Sanjuán Portugal FJ, Naya Manchado J, Alfaro Alfaro MJ. [Usefulness of MBDS in detection of adverse drug events]. An Med Interna. 2007 Mar;24(3):113-119. PubMed; PMID 17590131.
18. Corral Baena S, Guerrero Aznar MD, Beltrán García M, Salas Turrens J. [Use of MBDS as a tool for the detection of drug-related adverse events]. Farm Hosp. 2004 Aug;28(4):258-265. PubMed; PMID: 15369436.
19. Zapatero Gaviria A, Barba R, Ruiz Giardin JM, Emilio Losa Garcia J, Marco Martinez J, Plaza Canteli S, et al. [Adverse drug events in patients hospitalized in internal medicine]. Rev Clin Esp. 2010 Jun;210(6):263-269. PubMed; PMID 20434147.
20. Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. 2001 Sep;10(5):407-410. PubMed; PMID11802586.
21. Van der Hooft CS, Sturkenboom MC, van Grootheest K, Kingma HJ, Stricker BH. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf. 2006;29(2):161-8. PubMed; PMID 16454543.
22. Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385-96. PubMed; PMID 16689555.
23. Trifirò, G., Gini, R., Barone-Adesi, F. et al. The Role of European Healthcare Data-bases for Post-Marketing Drug EffectivJanss, Safety and Value Evaluation: Where Does Italy Stand? Drug Saf. 42, 347-363 (2019). PubMed; PMID: 30269245.
24. Sorensen HT, Sabroe S, Olsen J. A framework for evaluation of secondary data sources for epidemiological research. Int J Epidemiol. 1996-04;25(2):435-442.PubMed; PMID 9119571.
25. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 200504;58(4):323-337. PubMed; PMID 15862718.
26. EMA. European public assessment report (EPAR) for Kaletra: Product information [Internet]. London: EMA; 2009 Sep- [updated 2020 Nov, access 2021 Jan]; Availa-ble from: https://www.ema.europa.eu/en/medicines/human/EPAR/kaletra.
27. AEMPS [Internet]. Label Fortecortín. Madrid: Ministry of Health: AEMPS; 2008 Sep [updated 2017 May, access 2021 Jan]. Available from: https://cima. aemps.es/cima/.
28. EMA. European public assessment report (EPAR) for Tresiba: Product information [Internet]. London: EMA; 2013 Feb- [updated 2020 Nov, access 2021 Jan]; Available from: https://www.ema.europa.eu/en/medicines/human/.
29. AEMPS [Internet]. Label Dolquine. Madrid: Ministry of Health: AEMPS; 2011 Oct [updated 2018 Sep, access 2021 Jan]. Available from: https://cima.aemps. es/cima/dochtml/ft/74904/FT_74904.html.
30. Crescioli G, Brilli V, Lanzi C, Burgalassi A, Ieri A, et al. Adverse drug reactions in SARS-CoV-2 hospitalised patients: a case-series with a focus on drug-drug interactions. Intern Emerg Med. 2020 Dec 23:1-14. PubMed; PMID 33355896.
31. EMA. European public assessment report (EPAR) for Rebif: Product information [Internet]. London: EMA; 2009 Oct- [updated 2021 Jan, access 2021 Jan]; Available from: https://www.ema.europa.eu/en/medicines/human/.
32. AEMPS [Internet]. Label Clexane. Madrid: Ministry of Health: AEMPS; 1989 Oct [updated 2020 May, access 2021 Jan]. Available from: https://cima.aemps. es/cima/dochtml/ft/58502/FT_58502.html.
33. Méndez Fernández R, Graña López L, Rodríguez González R. [Adverse reactions to contrast media]. In: Martí-Bonmatí L, Pallardó Calatayud Y, editors. [Contrast media in Radiology]. SERAM Monograph. Madrid: Medica Panamericana; 2008. p. 115-127.
34. ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media. Washington (USA): ACR;2020. Available from: https://www.acr.org/Clinical-Resources/Contrast-Manual.
35. Kang SK. Diagnosis and treatment of an acute reaction to a radiologic contrast agent [Internet]. Waltham, MA: UpToDate; 2020 Dec [updated 2019 Apr, access 2021 Jan]. Available from: https://www.uptodate.com/.
36. EMA. European public assessment report (EPAR) for RoActemra: Product information [Internet]. London: EMA; 2010 Jan- [updated 2020 Sep, access 2021 Jan]; Available from: https://www.ema.europa.eu/en/medicines/human/.
37. AEMPS [Internet]. Label Zitromax. Madrid: Ministry of Health: AEMPS; 1996 Sep [updated 2020 Jun, access 2021 Jan]. Available from: https://cima.aemps. es/cima/.
38. AEMPS [Internet]. Label Augmentine 875 mg/125 mg comprimidos recubiertos con película. Madrid: Ministry of Health: AEMPS; Feb 1993 [updated 2018 Feb, access 2021 Jan]. Available from: https://cima.aemps.es/cima/.
39. AEMPS [Internet]. Label Codeisan. Madrid: Ministry of Health: AEMPS; 1940 Dic [updated 2018 Jun, access 2021 Jan]. Available from: https://cima.aemps. es/cima/.
40. Cuker A, Peyvandi F. Coronavirus disease 2019 (COVID-19): Hypercoagulability [Internet]. Waltham, MA: UpToDate; 2020 Dec [updated 2021 Jan, access 2021 Jan]. Available from: https://www.uptodate.com.
41. Kim AY, Gandhi RT. Coronavirus disease 2019 (COVID-19): Management in hospitalized adults [Internet]. Waltham, MA: UpToDate; 2020 Dec [updated 2020 Dec, access 2021 Jan]. Available from: https://www.uptodate.com.
42. Center for the Coordination of Health Alerts and Emergencies, editor. [Technical document: Clinical management of COVID-19 – hospital care]. [Internet]. Madrid: Ministry of Health; 2020 Jun. Available from: https://www.mscbs.gob. es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos.htm.
43. AEMPS, editor. [Treatments available subject to special access conditions for the management of respiratory infection by SARS-CoV-2]. [Internet]. Madrid: Ministry of Health: AEMPS; 2020 Mar- [updated 2020 Jul; access 2021 Jan]. Available from: https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid%E2%80%9119/.
44. Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: An overview. Eur J Pharmacol. 2020 Dic; 889:173644. PubMed; PMID: 33053381.
45. IDSSA. Guidelines on the Treatment and Management of Patients with COVID-19 v.4.0.4. Washington (USA): IDSSA;2021 Feb. Available from: https://www.idsociety.org/COVID19guidelines.
____