Moreno-Ramos C1, Gil-Sierra MD1,2, Briceño-Casado MP3, Ríos-Sánchez E1
1 Servicio de Farmacia Hospitalaria. Hospital Universitario Puerto Real. Cádiz (España)
2 Departamento de Farmacología. Universidad de Sevilla. Facultad de Farmacia. Sevilla (España)
3 Servicio de Farmacia Hospitalaria. Hospital Universitario de Jerez de la Frontera. Jerez de la Frontera (España)
Fecha de recepción: 04/06/2022 – Fecha de aceptación: 27/06/2022
Correspondencia: María del Pilar Briceño Casado – Hospital Universitario de Jerez de la Frontera (Servicio de Farmacia Hospitalaria) – Ronda de Circunvalación, s/n – 11407 Jerez de la Frontera, Cádiz (España)
pilarbricenocasado@gmail.com
____
SUMMARY
Objetive: Tumour necrosis factor (TNF) is a relevant therapeutic target in the treatment of inflammatory diseases. The objective was to determine the influence of the effect of a first-line anti-TNF regimen on the effectiveness of adalimumab, as well as to obtain real long-term data.
Methods: Retrospective descriptive study was conducted between January 2013 and July 2021. Patients diagnosed with ulcerative colitis treated with adalimumab who previously received at least one anti-TNF agent were included. Mayo Clinic Score up to 72 months was measured as effectiveness endpoint.
Results: Thirty-one patients were included. More than a third of patients treated with adalimumab as second-line anti-TNF had primary non-response. More than half of the patients treated with adalimumab presented clinical remission or response at 6 months of treatment, decreasing to one-tenth at 72 months.
Conclusion: The use of two anti-TNF therapy could be a strategy with acceptable effectiveness and favorable efficiency.
Key words: Ulcerative colitis, tumour necrosis factor inhibitors, adalimumab, treatment failure, long term effect.
Influencia de la terapia anti-TNF de primera línea en la efectividad de adalimumab en colitis ulcerosa: resultados de práctica clínica a largo plazo
RESUMEN
Objetivo: El factor de necrosis tumoral (TNF) es una diana terapéutica relevante en el tratamiento de enfermedades inflamatorias. El objetivo fue determinar la influencia del efecto de una terapia anti-TNF de primera línea sobre la eficacia de adalimumab, así como obtener datos reales a largo plazo.
Métodos: Estudio descriptivo retrospectivo realizado entre enero de 2013 y julio de 2021. Se incluyeron pacientes diagnosticados de colitis ulcerosa tratados con adalimumab que recibieron previamente al menos un agente anti-TNF. Como criterio para la valoración de la eficacia se usó Mayo Clinic Score hasta 72 meses.
Resultados: Treinta y un pacientes fueron incluidos. Más de un tercio de los pacientes tratados con adalimumab como anti-TNF de segunda línea tuvieron un fallo primario. Más de la mitad de los pacientes tratados con adalimumab presentaron remisión clínica o respuesta a los 6 meses de tratamiento, descendiendo a una décima parte a los 72 meses.
Conclusiones: El uso de dos terapias anti-TNF podría ser una estrategia con una efectividad aceptable y una eficiencia favorable.
Palabras clave: Colitis ulcerosa, inhibidor del factor de necrosis tumoral, adalimumab, fallo terapéutico, efecto a largo plazo.
____
INTRODUCTION
Ulcerative colitis is a chronic disease characterized by inflammation and ulceration of colon and rectum. Some symptoms are diarrhoea and abdominal pain. The incidence and prevalence in the Western countries increased in recent years, reaching 8/100,000 inhabitants in Spain1. Tumour necrosis factor (TNF) is a proinflammatory cytokine involved in the pathogenesis of the disease. TNF inhibitors (anti-TNF) –such as infliximab and adalimumab– prevent the binding of TNF with its receptor, avoiding the triggering of the inflammatory process2,3. The use of these drugs in ulcerative colitis controls the disease progression in many patients. However, no clinical improvement is obtained in other patients. This treatment failure can occur at the start of the anti-TNF regimen (primary non-response) or after having achieved a primary response (secondary non-response). A common strategy for treating ulcerative colitis is the sequential use of a second anti-TNF agent when the first drug fails. However, previous studies have suggested an insufficient response of the second anti-TNF regimen after failed therapy with the first anti-TNF scheme4. It is important to generate evidence on the relationship between different types of therapeutic failures of anti-TNF drugs in their sequential use. The objective was to evaluate the influence of the effect of a first-line anti-TNF regimen on adalimumab, in addition to obtaining real long-term effectiveness data.
METHODS
Retrospective descriptive study included patients diagnosed with ulcerative colitis pre-treated with a prior anti-TNF agent from January 2013 to July 2021. The standard dose of adalimumab used was 80 mg at week 0, followed by 40 mg at week 2, and 40 mg every two weeks subsequently. The adalimumab dosage of 160 mg at week 0, followed by 80 mg at week 2, and 40 mg every two weeks subsequently was used in patients in need of achieving a faster response. The Dominion Farmatools® software and digital medical records were used to collect patient data.
The effectiveness of adalimumab was assessed using the Mayo Clinic Score (MCS) at 6, 12, 24, 36, 48, 60, and 72 months of treatment. This index assesses stool frequency, rectal bleeding, mucosa appearance at endoscopy, and general patient evaluation. The score ranges from 0 to 125. Clinical remission (R) was defined as an MCS value ≤2. The clinical response (CR) was considered as a decrease of ≥3 points in MCS with respect to baseline value. The lack of clinical response (LOR) was defined as the absence of any of the above situations. Those patients with LOR and treatment suspension in a certain week were categorized as LOR patients in subsequent weeks. The influence of the effect of the first anti-TNF drug was estimated by the association between the primary and secondary non-responses of the first and second anti-TNF agents. Primary non-response to anti-TNF therapy was considered as LOR in the induction period (before week 10 for infliximab and before week 4 for adalimumab). Secondary non-response to anti-TNF treatment was defined as LOR after the induction period. The results obtained were reviewed by two pharmacists, discussing the possible discrepancies found and reaching a consensus.
RESULTS
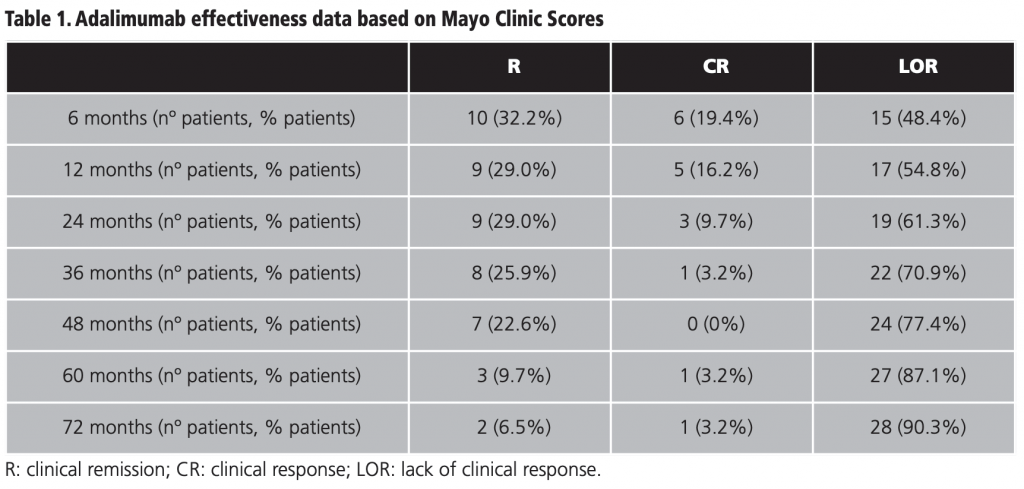
Thirty-one patients were included. With respect to gender, 55% were women and 45% men. The median age was 43 (21-86) years. The most frequently administered anti-TNF agents in the first line of biological treatment –previously to adalimumab– were infliximab (77,4%) and golimumab (3,2%). The median duration of treatment with adalimumab was 18 (1-91) months. The baseline MCS value of patients presented a median of 8 (2-12). Table 1 shows the effectiveness data of adalimumab according to MSC score at 6, 12, 24, 36, 48, 60 and 72 months of therapy. The number of patients with R decreased progressively over time, from 32,2% of patients in R at 6 months to 6,5% of cases at 72 months. At 6 months, 51,6% of the patients achieved R or CR. This effectiveness progressively decreased up to 72 months, when we found 9,7% of patients with R or CR.
Regarding the dosage regimen used in the induction phase, 90,3% of the patients received 160 mg of adalimumab in week 0, followed by 80 mg in week 2, and subsequently 40 mg every two weeks. In the maintenance phase, 9,7% of the patients required intensification of the drug, administering 40 mg weekly instead of every 14 days.
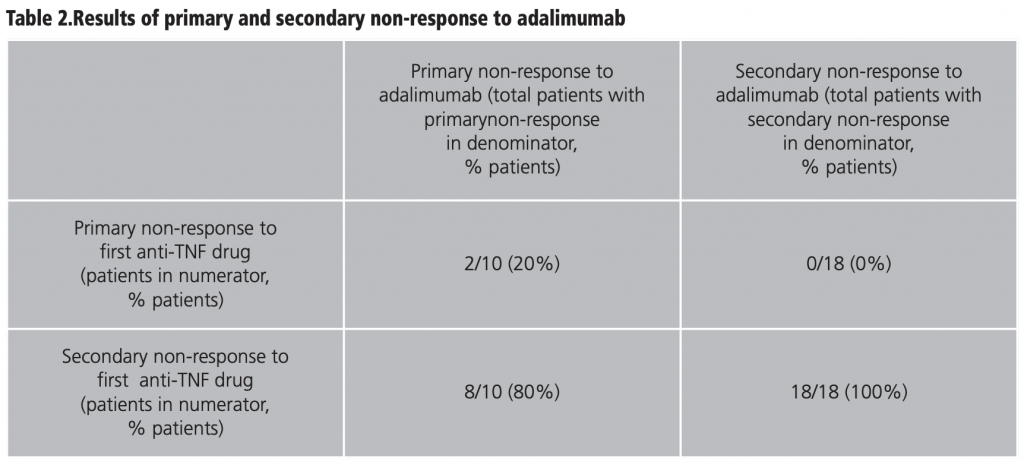
Table 2 shows the results of primary and secondary non-response to adalimumab at 72 months, with respect to the type of failure presented to the first anti-TNF agent. Ten patients (32,3%) showed primary non-response to adalimumab, of which 2 cases presented primary non-response to the previous anti-TNF drug and 8 had secondary non-response to the first anti-TNF agent. On the other hand, 18 (58,1%) patients with prior secondary non-response to anti-TNF therapy showed secondary non-response to adalimumab.
DISCUSSION
The sequential use of two anti-TNF agents is a widely used strategy. There is literature indicating the loss of efficacy of an anti-TNF drug in 20-50% of patients during the first 12 months and recommending switching to another agent of the same group6. Systematic reviews with meta-analyses report a mean R rate of 45% of patients at 24 months after switching to a second anti-TNF drug7. Less effectiveness was found in our population, since 29% of cases reached R at 24 months. The effectiveness of second anti-TNF agent could be related to the reason for the switch in treatment. The 20% of our patients with primary non-response to the first anti-TNF therapy subsequently had primary non-response to adalimumab. Likewise, no patients with secondary non-response to adalimumab had presented primary non-response to the first anti-TNF regimen. Considering our limited sample size, it could also be thought that there is a greater proportion of patients with primary non-response to adalimumab who had secondary non-response to the first line of anti-TNF therapy because there are simply fewer patients with primary non-response at the first anti-TNF agent in the global population.
Almost 50% of our patients demonstrated R or CR in the first 12 months of treatment with adalimumab after a prior anti-TNF agent. As indicated in some reviews based on clinical practice, some patients previously exposed to a first anti-TNF therapy required an increase in dose of adalimumab ‒both during induction and maintenance periods‒ in contrast to naïve patients8,9. This finding may be due to a phenomenon of immunogenicity10. Other studies pointed to short-term re-induction of the drug to regain treatment response as an intensification practice11. In our study, 90,3% of patients started with 160 mg at week 0, followed by 80 mg at week 2, and then 40 mg every two weeks. It is important to generate evidence to decrease the probability of two sequential primary non-responses. Perhaps intensifying the treatment regimen could decrease this probability. Furthermore, the response to a second anti-TNF agent could occur later, such that a 4-week interval may be short to assess possible primary failures11.
There are few studies involving patients routinely found in this clinical practice context and with an extensive follow-up period like our work. On the other hand, our data review was conducted by two pharmacists. This could reduce subjectivity in the interpretation of the extracted data. The main limitations of this study are the small sample size and the study design. The lack of randomization and a control group does not allow us to reliably confirm whether results observed may be due to the disease evolution itself or to a sequencing strategy of two anti-TNFs agents. Consequently, the data should be interpreted with caution and considering the available information in clinical trials.
In the current context, adalimumab biosimilars have extensive experience of use in real clinical practice and clinical trials found adequate efficacy and safety12,13. Biosimilar drugs are subject to control standards that guarantee quality, safety and efficacy comparable to reference drugs. Its introduction in the market (after patents expiry) and consequent reduction in prices have allowed a very favorable impact on pharmaceutical spending for public administrations14. Therefore, the use of two biosimilar anti-TNF agents sequentially could be an efficient strategy that favors the sustainability of national health systems.
CONCLUSION
In conclusion, the use of adalimumab as second anti-TNF drug administered sequentially reported more than one third of cases with primary drug non-response. About 90% of our patients required dose intensification of adalimumab as second anti-TNF therapy. Patients treated with adalimumab in R or CR were more than half at 6 months of treatment, progressively decreasing to one-tenth at 72 months.
Conflict of interest: Gil-Sierra MD participated in an advisory board of Janssen Pharmaceutica. The rest of the authors have no conflicts of interest.
BIBLIOGRAFÍA
1. Chaparro M, Garre A, Nuñez Ortiz A. et al. Incidence, clinical characteristics and management of inflammatory bowel disease in Spain: large-scale epidemiological study. J Clin Med 2021; 10(13): 2885. Doi: 10.3390/jcm10132885
2. European Medicines Agency. Summary of product characteristics of Remicade. [internet]. 2009. Available: https://www.ema.europa.eu/en/documents/ product-information/remicade-epar-product-information_en.pdf (accessed 13 jan 2022).
3. European Medicines Agency. Summary of product characteristics of Humira. [internet]. 2008. Available: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (accessed 13 jan 2022).
4. Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis. 2010;69 (5):817-21. Doi: 10.1136/ard.2009.112847.
5. Mayo Score/Disease Activity Index (DAI) for Ulcerative Colitis. MDCalc. 2016. Available in: https://www.mdcalc.com/mayo-score-disease-activity-index-dai-ulcerative-colitis#evidence (accessed 11 nov 2021).
6. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106(4):674-84. Doi: 10.1038/ajg.2011.60.
7. Gisbert JP, Marín AC, Mcnicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41(7):613-23. Doi: 10.1111/apt.13083.
8. Iborra M, Pérez-Gisbert J, Bosca-Watts MM, et al. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol. 2017;52(7):788-99. Doi: 10.1007/s00535-016-1274-1.
9. Ma C, Panaccione R, Heitman SJ, et al. Systematic review: the short-term and long-term efficacy of adalimumab following discontinuation of infliximab. Aliment Pharmacol Ther. 2009;30(10):977-86. Doi: 10.1111/j.1365-2036. 2009.04101.x.
10. Moss AC, Brinks V, Carpenter JF. Review article: immunogenicity of anti-TNF biologics in IBD-the role of patient, product and prescriber factors. Aliment Pharmacol Ther. 2013;38(10):1188-97. Doi: 10.1111/apt.12507.
11. Srinivasan A, Vasudevan A, McFarlane A, et al. Anti-TNF re-induction is as effective, simpler, and cheaper compared with dose interval shortening for secondary loss of response in Crohn’s disease. J Crohns Colitis. 2018;12(3):280-8. Doi: 10.1093/ecco-jcc/jjx144.
12. Chandra A, Kanth R, Thareja S. Efficacy and safety of adalimumab biosimilar (Exemptia) in moderate to severe steroid refractory ulcerative colitis patients: real life outcomes in resource constrained setting at 24 weeks follow up. Biologics. 2019;13:191-200. Doi: 10.2147/BTT.S214518.
13. Frampton, JE. SB5: An Adalimumab Biosimilar. BioDrugs. 2018;32(5): 507-10. Doi: 10.1007/s40259-018-0307-0.
14. Villamañan E, González D, Armada, et al. Juego de patentes. Sobre medicamentos genéricos y biosimilares [The patents game. Generic and biosimilar drugs]. Rev Calid Asist. 2016;31:99-105. Doi: 10.1016/j.cali.2015.08.002.
____