Borrás-Blasco J1, Cornejo S1, Cortes X2, Sanchis L2, Casterá E1
- Pharmacy Service. Hospital Sagunto. Spain.
- Gastroenterology Service. Hospital Sagunto. Sagunto Spain
Fecha de recepción: 31/08/2023 – Fecha de aceptación: 18/09/2023
Correspondencia: Joaquín Borrás-Blasco. Pharmacy Department. Hospital de Sagunto. Avda Ramon y Cajal s/n Sagunto 46520, Valencia · Email: jborrasb@gmail.com
____
Objective: To explore persistence, retention rate, safety and serum levels after switching from CT-P13IV to CT-P13SC in patients receiving escalated CT-P13IV dosing frequency. Methods: This is a retrospective cohort study. The primary outcome was treatment persistence after switch at latest follow-up. We recorded number of administrations for IV per patient/year. In patients with CT-P13SC, we recorded the number of outpatient hospital pharmacy visits/dispensation per patient/year. Infliximab trough levels were measured prior and 6 months after switching.
Results: We included 11 patients who were at least 6 months with intensified Infliximab CT-P13IV 5mg every 4 or 6 weeks. All patients had their IBD controlled and 10 took concomitant immunosuppressive therapy. After at least 8 months on CT-P13SC, 10 [90.9%] patients continued and 1 patient [9.1%] stopped due to disease relapse. Previous to switch, CT-P13IV persistence rate was 3,78 ± 2.46 years. After switch, CT-P13SC was 0,98 ± 0.41 years. The retention rate at 10 months was 91%. During the study period, any CT-P13SC intensification was made and 7 patients suspended immunosuppression. Median infliximab levels prior to switch was 8.2 μg/dl [2.7-21.3] and 13.5 μg/dl [8.2-23.1] after 6 months of switching. No antibodies to CT-P13SC were detected. Median number of hospital of day visits per patient/year for CT-P13IV were 8. The number of outpatient hospital pharmacy visits/dispensation for CT-P13SC patient/year were 6. No safety findings were recorded.
Conclusion: Among patients on infliximab CT-P13 IV with intensified therapy switched to CT-P13SC, we observed high treatment persistence, retention rate and low immunogenicity, with no clinical changes in disease activity.
Keywords: Persistence, Inflammatory bowel disease, Infliximab CT-P13 intravenous, Infliximab CT-P13 subcutaneous, Retention Rate
Persistencia, tasa de retención y niveles séricos de infliximab ct-p13 subcutáneo tras el cambio de infliximab ct-p13iv intensificado en pacientes con enfermedad inflamatoria intestinal.
Objetivo: Explorar la persistencia, tasa de retención, seguridad y niveles séricos tras cambiar de Infliximab CT-P13IV a CT-P13SC en pacientes con intensificación de CT-P13IV. Métodos: Estudio de cohorte retrospectivo. El objetivo primario es la persistencia del tratamiento tras cambio de via. Se registraron las administraciones intravenosas por paciente/año y las visitas/dispensaciones a farmacia hospitalaria por paciente/año para recoger CT-P13SC. Los niveles de infliximab se midieron antes y 6 meses tras cambio.
Resultados: Se incluyeron 11 pacientes con al menos 6 meses con CT-P13IV intensificado 5 mg cada 4 o 6 semanas. Todos los pacientes tenían su EII controlada y 10 recibían inmunosupresores concomitantemente. Tras al menos 8 meses con CT-P13SC, 10 [90,9 %] pacientes continuaban y 1 paciente [9,1 %] suspendió por una recaída. Inicialmente, la persistencia de CT-P13IV era 3,78 ± 2,46 años. Después del cambio a CT-P13SC fue de 0,98 ± 0,41 años. La tasa de retención a los 10 meses fue del 91%. Durante el período de estudio no se intensificó ningún CT-P13SC y 7 pacientes suspendieron la inmunosupresión. La mediana de niveles de infliximab inicial fue 8,2 μg/dl [2,7-21,3] y 13,5 μg/dl [8,2-23,1] 6 meses tras el cambio, sin detección de anticuerpos contra CT-P13SC. La mediana de visitas al hospital por paciente/año para CT-P13IV fue 8. Las visitas/dispensaciones a farmacia hospitalaria paciente/año de CT-P13SC fue 6. No hubo hallazgos de seguridad.
Conclusión: Los pacientes con pauta infensificada de CT-P13 IV que cambiaron a CT-P13 SC, tuvieron alta persistencia al tratamiento, alta tasa de retención y baja inmunogenicidad, sin cambios clínicos de la enfermedad.
Palabras clave: Persistencia, Enfermedad inflamatoria intestinal, Infliximab CT-P13 intravenoso, Infliximab CT-P13 subcutáneo, Tasa de retención
____
Introduction
Inflammatory bowel disease (IBD) is a heterogeneous group of chronic inflammatory disorders: the main phenotypes comprise ulcerative colitis (UC) and Cohn’s disease (CD). IBD is characterized by chronic relapsing intestinal inflammation. Although the etiology of IBD remains largely unknown, it involves factors as genetics, environmental, microbial and the immune-responses1. Infliximab is the most widely studied and used anti-tumor necrosis factor biologic for therapy of chronic immune-mediated diseases2. It is a chimeric human-murine monoclonal antibody, which has now been licensed for use in many conditions including IBD. Its use in IBD has been shown to reduce hospitalization and rates of surgery in clinical trials3.
Biosimilars are drugs, which are comparable to the originator biologics in terms of mechanism of action as well as efficacy and safety4. Infliximab CT-P13 is a biosimilar version of infliximab and has shown to be efficacious in patients with IBD3. Infliximab CT-P13 is available in Intravenous (IV) and a new subcutaneous formulation of the infliximab Biosimilars CT-P13 has recently been developed subcutaneous (SC). Infliximab CT-P13 SC provides similar response rates to intravenous treatment in IBD patients5,6. Infliximab CT-P13 SC has demonstrated an improved pharmacokinetic profile compared with IV infliximab: the more stable exposure and increased systemic drug concentrations A post hoc analysis of pivotal data in patients with RA and CD also demonstrated significantly lower immunogenicity for patients receiving CT-P13 SC than those receiving CT-P13 IV (p < 0.0001)7.
Adherence to treatment is defined as taking the medication by the patient as prescribed8. There are multiple options to measure adherence; however, none of them have good sensitivity in isolation. Lately persistence term is used to complete adherence. Persistence, an easily measured indicator of the long-term therapeutic benefit of a drug, is defined as “the duration of time from initiation to discontinuation of therapy”9.
Infliximab CT-P13 IV therapy intensifications, both those based on shortening the dosing interval and those involving the standard dose are common in IBD patients. In the case of shortening of the dosing interval produces an increase of patient hospital visits to receive Infliximab CT-P13 IV that has implications for healthcare services and patients’ quality of life. For these patients, the availability of Infliximab CT-P13 SC could be a solution because of offers a significant patient time saving, reduce patient visits and pressure on IBD infusion units. Limited real-world data are available on switching from Infliximab CT-P13 IV to Infliximab CT-P13 SC in patients receiving intensified Infliximab CT-P13 IV dosing frequency. The objective of this study was to explore the persistence, retention rate, safety and serum levels after switching from Infliximab CT-P13 IV to Infliximab CT-P13 SC in patients receiving escalated Infliximab CT-P13 IV dosing frequency.
Material And Methods
We conducted an observational, retrospective and descriptive study of IBD patients treated with escalated Infliximab CT-P13 IV dosing frequency, who switched to Infliximab CT-P13 SC pen from Oct 2021 to Jun 2023. Persistence was monitored following switch. Fecal calprotectin [FC] and C-reactive protein [CRP] were recorded at baseline and follow-up, if available. Infliximab trough levels were measured prior to switch and at month 6 following switch. Data on their age, weight, infliximab dosing, safety and retention rate were collected. The primary outcome measure was treatment persistence after switch at latest follow-up. Secondary outcome measures included number of administrations of Infliximab CT-P13 IV and Infliximab CT-P13 SC, dosing regimens, infliximab serum levels, safety, need for immunosuppressive therapy and adherence.
We assessed infliximab serum levels prior to and after switch. Serum infliximab levels and antibodies to infliximab were measured using a drug-sensitive assay; a previously validated in-house ELISA was used to measure infliximab levels and neutralising antibodies. Positive antidrug antibody with the drug-sensitive assay was defined as an antibody titre of 1:40 or greater.
For the period of patients with Infliximab CT-P13 IV, we recorded dosing regimen, number of administrations for IV, number of hospital of day visits per patient/year. Infliximab CT-P13 IV adherence data was obtained from the Pharmacy Department’s IV therapy preparation and validation software (Oncofarm® IMF). For the period of patients with Infliximab CT-P13 SC, we recorded dosing regimen and the number of outpatient hospital pharmacy visits/dispensation per patient/year. We dispensed Infliximab CT-P13 SC for every two months. Treatment adherence was obtained from the dispensation records of the Hospital Pharmacy Department. Individualized Infliximab CT-P13 SC dispensations and correlated dates during the study period were collected using Outpatient Clinic Hospital Pharmacy software DISPENSA, (Oncofarm® IMF) which allows dispensing and follow-up of outpatient.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and after approval of the protocol and its amendments by the local Ethics Committee
Statistical analysis
Categorical data are expressed as absolute frequency and percentage, and continuous data as mean and standard deviation [SD]. Differences in the infliximab serum levels of Inflixamab prior to and after switch were analyzed with the Student’s T test for paired data. Statistical significance was set at p < 0.05. Statistical analysis was conducted using the SPPS 19.0 working package [SPSS Inc., Chicago, IL].
Result
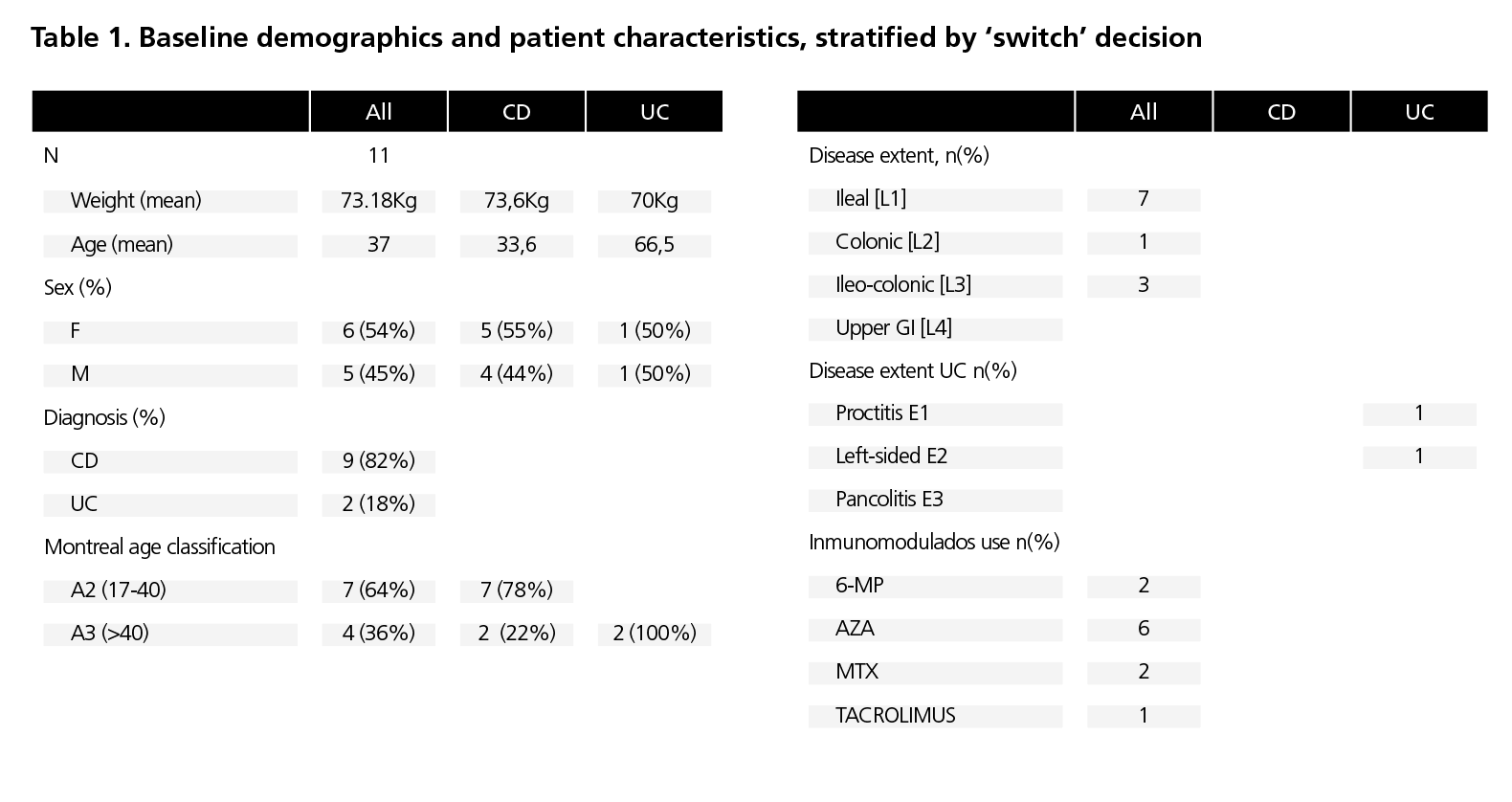
We included 11 patients, of whom 9 [82%] had CD and 2 [18%] UC with escalated Infliximab CT-P13 IV dosing frequency. Mean age was 37 years, and 6 (54%) patients were women. See table 1 for patient characteristics. All patients were at least 6 months with Infliximab CT-P13 IV previous to switching to Infliximab CT-P13 SC and were on 4 and 6-weekly dosing of 5 mg/kg Infliximab CT-P13 IV prior to switching. All had been treated with intensified doses of Infliximab CT-P13 IV for at least 6 months. At the time of the switch all patients had controlled their inflammatory bowel disease and 10 (91%) patients were receiving concomitant immunosuppressive therapy. After at least 6 months on Infliximab CT-P13 SC, 10 [90.9%] patients continued on Infliximab CT-P13 SC and 1 patient [9.1%] stopped treatment due to disease relapse. The patient switched back Infliximab CT-P13 IV and nowadays continued on Infliximab CT-P13 IV. Previous to switch Infliximab CT-P13 IV persistence rate was 3.78±2.46 years. After switch Infliximab CT-P13 SC persistence rate was 0.98±0.41 years. All these patients continued with the Infliximab CT-P13 SC 120mg/14d and it was possible to suspend the immunosuppression in 7 (64%) of them. During the study period, any Infliximab CT-P13 SC intensification of dose was made. There was no significant change in FC and C-reactive protein from baseline. Fecal calprotectin levels were 136.6±250,42 µg/g at the baseline and 158.5±96,68 µg/g at the end of follow-up. Median infliximab serum levels prior to switch was 8.2 μg/dl [range 2.7-21.3] and 13.5 μg/dl [range 8.2-23.1, p <0.05] after 6 months of switching. We did not detected any antibodies to Infliximab CT-P13 SC. Patient adherence to Infliximab CT-P13 SC was >90% during the period study. This assessment was based on individualized drug dispensations and correlated dates during the study period were collected from the Outpatient Clinic Hospital Pharmacy database. Throughout the study, all patients successfully self-administered Infliximab CT-P13 SC pen and consistently injected the full dose in each administration. The median number of hospital of day visits per patient/year was 8. The number of outpatient hospital pharmacy visits/dispensation per patient/year was 6. No safety findings were recorded during this study. No serious adverse effects were notified that conditioned the discontinuation of the Infliximab CT-P13 SC.
Discussion
Infliximab CT-P13 SC offers more choices to clinicians and patients already established on intravenous infliximab. It provides a useful alternative for patients requiring these treatments, with the possibility of self-administration at home. Decisions to switch IV to Infliximab CT-P13 SC may be driven by several factors as capacity issues in infusion unit or in external pharmacy, patient preference or cost. The present real-world cohort study was conducted in 11 patients with IBD who were receiving intensified Infliximab CT-P13 IV and switch to Infliximab CT-P13 SC with the label dose showed that the real world elective switch has demonstrated to be safe, with no serious adverse events reported, maintaining clinical remission as well as a high degree of treatment persistence in more than 90% of the IBD patients switched. A possible reason for such a high level of persistence may be the higher therapeutic drug levels or the reduced immunogenicity that we found in our cohort.
As in other studies published to date10,11 in intensified patients, Infliximab serum levels increased significantly after 6 month of Infliximab CT-P13 SC. Although this increase, we did not observe toxicity. Besides, we found no immunogenicity problems in patients treated with Infliximab CT-P13 SC. The rates of immunogenicity were lower among Infliximab CT-P13 SC patients in our cohort compared with previously reported IV infliximab cohorts12,13. Drug levels in our study were obtained 24h before administration. However, in Infliximab CT-P13 SC it has been observed that drug levels remain stable during 14 days between doses, so determination would not be subject to trough levels as occurs with intravenous dosing14. Stable infliximab levels may be protective against development of immunogenicity. In our study, we observed low rates of immunogenicity and higher infliximab serum levels post-switch. Higher levels of Infliximab have been related with a better mucosal healing15,16. The association of immunosuppressive and anti-TNF drugs has been associated with an increased risk of infections and other adverse effects17,18. In our study we observed that patients could decrease inmunosupressants after switching, this could be possible as a result of the maintained disease activity and higher levels of infliximab with Infliximab CT-P13 SC. Our findings corroborate evidence from earlier cohorts demonstrating significant decrease inmunosupressants after switching from Infliximab CT-P13 IV to Infliximab CT-P13 SC11,19.
In COVID era, to avoid visits to hospital is likely to reduce the risk of transmission of the infection, which could subsequently lead to many health benefits. It has effectively minimized the need for frequent hospital visits and eliminated the necessity for patients to travel to the hospital as it is possible to dispense medication for 2 or 3 months. Moreover, it ensures treatment continuity, pharmaceutical care, and promotes safe self-administration of biological drugs.
The present study was conducted under conditions of daily clinical practice. However, it is important to interpret the results considering some limitations due to the small number of patients included. Further studies with more patients are needed to detect the clinical relevance of these findings.
Conclusion
Our real-world data support the feasibility of an elective switch to Infliximab CT-P13 SC in patients on infliximab CT-P13 IV with intensified therapy. We observed high treatment persistence, retention rate and low immunogenicity, with no change in clinical disease activity and infliximab CT-P13SC label dose. For this population, Infliximab CT-P13 SC offers advantages in terms of therapeutic flexibility and less dependence on infusion centers.
Conflicts of interest: The authors declare that they have no
conflicts of interest.
Bibliography
- Zhang YZ, Li YY. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99.
- Al Sulais E, AlAmeel T. Biosimilars to antitumor necrosis factor agents in inflammatory bowel disease. Biologics. 2020;14:1–11.
- Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, A N Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Cohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2017;45:3–13.
- Valcuende-Rosique A, Borrás-Blasco J, Martínez-Badal S, Cortes X, Aparicio-Rubio C, Casterá-Melchor E. Evaluation of persistence, retention “rate” and prescription pattern of original infliximab and infliximab CT-P13 in biologic-naïve patients with ulcerative colitis. Farm Hosp. 2022;46:296-300.
- Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A , Gawdis-Wojnarska B, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology 2021;160:2340–53.
- Verma AM, Patel A, Subramanian S, Subramanian S, Smith PJ. From intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: a pandemic-driven initiative. Lancet Gastroenterol Hepatol 2021;6:88–9.
- Yoo D, Westhovens R, Ben-Horin S, Reinisch W, Schreiber S, Ye B.B et al. Development of a subcutaneous formulation of CT-P13 (infliximab): maintenance subcutaneous administration may elicit lower immunogenicity compared to intravenous treatment. Arthritis Rheumatol. 2018;70(Suppl 9):2791–2792.
- Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691-705.
- De la Cueva Dobao P, Notario J, Ferrándiz C, López Estebaranz JL, Alarcón I, Sulleiro S, et al. Expert consensus on the persistence of biological treatments in moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2019;33:1214-223.
- Schreiber S, Ben-Horin S, Leszczyszyn J, Dudkowiak R, Lahat A, Gawdis-Wojnarska B, et al. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2340–2353.
- Smith PJ, Critchley L, Storey D, Gregg B, Stenson J, Kneebone A, et al. Efficacy and safety of elective switching from intravenous to subcutaneous infliximab [CT-P13]: A multi-centre cohort study. J. Crohn’s Colitis 2022 ;16:1436-1446.
- Ungar B, Chowers Y, Yavzori M, Picard O, Fudim E, Har-Noy O, et al. The temporal evolution of antidrug antibodies in patients with infammatory bowel disease treated with infiximab. Gut 2014;63:1258–64
- Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-tnf treatment failure in anti-tnf-naive patients with active luminal Cohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 2019;4:341–53
- Roblin X Veyrard P, Bastide L, Berger AE, Barrau M, Paucelle AS, et al. Subcutaneous injection of infliximab CT-P13 results in stable drug levels within 14-day treatment cycle in Cohn’s disease. Aliment. Pharmacol. Ther. 2022;56; 78–83.
- Annese V, Nathwani R, Alkhatry M, Al-Rifai A, Al Awadhi S, Georgopoulos F, et al. Optimizing biologic therapy in inflammatory bowel disease: A Delphi consensus in the United Arab Emirates. Therap. Adv. Gastroenterol. 2021:14; 17562848211065329
- Ungar B, Levy I, Yavne Y, Yavzori M, Picard O, Fudim E, et al. Optimizing Anti-TNF-α Therapy: Serum Levels of Infliximab and Adalimumab Are Associated With Mucosal Healing in Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2016;14:550–557.e2.
- Hindryckx P, Novak G, Bonovas S, Peyrin-Biroulet L, Danese S. Infection Risk With Biologic Therapy in Patients With Inflammatory Bowel Disease. Clin. Pharmacol. Ther. 2017;102:633–41.
- Queiroz N.S.F, Regueiro M. Safety considerations with biologics and new inflammatory bowel disease therapies. Curr. Opin. Gastroenterol. 2020;36:257–64.
- Chivato I, Saiz RM, Vicente B, Revilla N, Andrés L, Pachón C, et al. High Efficacy of Switch to Subcutaneous Infliximab in Patients with Inflammatory Bowel Disease Under Intensified Intravenous Infliximab Treatment: One-Year Results. Gastroenterology and Hepatology 2023;46: S121.
____
