Luque Calvo, C.1; Romero Garrido, JA.2,3; Benedí González, J3
- Complutense University of Madrid, Spain.
- Department of Pharmacy. University Hospital La Paz, Madrid, Spain.
- Department of Pharmacology (Pharmacognosy and Experimental Pharmacology), Complutense University of Madrid, Spain (UCM).
Fecha de recepción: 17/03/2023 – Fecha de aceptación: 18/04/2023
Mail: carolinaclc6@gmail.com
____
Abstract
Introduction: The aim of this study is to assess the economic burden of clotting factors for haemophilia therapy in Madrid, Spain.
Materials and Methods: The epidemiology included was based on the consumption of the clotting factors, exclusively used in haemophilic patients, and the costs of these, excluding indirect costs derived from the disease, from a hospital of reference in Madrid, and distributed by age, diagnosis, type of treatment (on demand or prophylactic) and the presence of bypass agents on their treatment.
Results and discussion: A total of 701 patients were included in the retrospective study during from 2011 until 2020. Consumption and costs were higher in patients with haemophilia A, and for the subgroups studied, those patients with high severity and presence of bypassing agents, the amount of coagulation factor (measured in International Units) and costs (in euros), were the ones with higher weight. When comparing the consumption of Madrid’s population with other European or American patients, data is found out to be similar.
Conclusion: The conclusion after this observational study is that patients with haemophilia have a great impact on the health expense in the Community of Madrid, given the fact that only direct costs have been considered, being more acute as results show, in haemophila A, younger patients, severe disease and presence of inhibitors.
Keywords: haemophilia, coagulation factors, consumption, costs, burden, Spain.
Impacto farmacoeconómico de la hemofilia en Madrid, España: análisis de 10 años.
Introducción: El objetivo de este estudio es evaluar la carga económica de factores de coagulación para la terapia de la hemofilia en Madrid, España.
Materiales y métodos: La epidemiología incluida se basó en el consumo de los factores de coagulación, uso exclusivo en pacientes hemofílicos, y los costes de los mismos excluyendo los costes indirectos derivados de la enfermedad, de un hospital de referencia de Madrid, y distribuidos por edad, diagnóstico, tipo de tratamiento (a demanda o profiláctico) y presencia de anticuerpos inhibidores en su tratamiento.
Resultados y discusión: Un total de 701 pacientes fueron incluidos en el estudio retrospectivo desde 2011 hasta 2020. El consumo y los costes fueron mayores en los pacientes con hemofilia A, y para los subgrupos estudiados, aquellos pacientes con alta gravedad y presencia de agentes bypass, la cantidad de factor de coagulación (medida en Unidades Internacionales) y los costes (en euros), fueron los de mayor peso. Al comparar el consumo de la población madrileña con otros pacientes europeos o americanos, se observa que los resultados son similares.
Conclusiones: La conclusión tras este estudio observacional es que los pacientes con hemofilia tienen un gran impacto en el gasto sanitario de la Comunidad de Madrid, dado que sólo se han considerado los costes directos, siendo mayores, en hemofilia A, pacientes más jóvenes, enfermedad grave y presencia de inhibidores.
Palabras clave: hemofilia, factores de coagulación, consumo, costes, carga, España.
____
Introduction
Haemophilia is a clinically relevant rare disease that can be classified in two types: haemophilia A (HA), which results from the deficiency or dysfunction of coagulation factor VIII (FVIII), and haemophilia B (HB), deficiency or dysfunction of factor IX (FIX). Both haemophilia A and B are due to mutations in genes located on chromosome X. Because of this, it largely affects males. The symptoms are bleeding-related symptoms, roughly proportional to the degree of factor deficiency in plasma. Factor IX is a vitamin K– dependent enzyme that is essential for normal thrombin generation 1–3.
The incidence of haemophilia A is approximately 1 in 5.000 new-borns (males) or 1 in 10.000 births. The prevalence of the disease varies according to country reports with a range of 5.4-14.5 cases per 100.000 males. In Spain, in 2010, the total number of patients with haemophilia A was 2,5954.
The worldwide prevalence of haemophilia B according to the World Federation of Haemophilia (WFH) data is approximately 28.430 cases, i.e., 0.04 cases per 10.000 population. The prevalence in Spain is estimated to be around 319 persons, representing 13.3% of haemophilia patients.
The ratio in Spain for haemophilia A and B is about 6,5:15
There are different degrees of haemophilia depending on the residual factor level in the blood:
mild haemophilia: the residual factor rate exceeds 5%
moderate haemophilia: the residual factor rate is between 1 and 5%, and
severe haemophilia: the rate is less than 1%6.
Bleeding is the main and best-known symptom of haemophilia and can occur anywhere in the body, with the severity depending on the location and degree of haemophilia. The signs of haemophilia A and B are the same7.
The treatments used are replacement of the factors that the patient lacks. Medicines containing these proteins are known as blood-derived medicines. Depending on the origin of the protein, they can be plasmatic or recombinant. Plasma medicines are obtained from donor plasma by plasma fractionation, and recombinant medicines are obtained by genetic engineering.
To achieve adequate peak and trough levels, with the administration of replacement therapy using plasma or recombinant factors as haemorrhage prophylaxis, infusions 3-4 times a week are required in severe haemophilia A and twice a week in haemophilia B8. The explanation to this is that the elimination half-life (t1/2) of most FVIII concentrates ranges from 12 to 20 hours and they are administered intravenously 2 to 4 times a week, which affects the quality of life of patients and represents a major problem for adherence to treatment9.
The different approaches to the treatment of these patients are either prophylactic or on-demand: administration of the drug continuously or only when bleeding must be stopped at a certain point in time because a haemorrhage has occurred, respectively. To this, we must add the Immune tolerance induction (ITI) therapy to those patients that need it10
The doses of each product for a treatment will depend on the weight of the patient and the severity of the haemophilia, and are calculated as follows, measured in International Units (IU)11:
IU required = body weight (kg) x desired increase in factor VIII (% or IU/dl) x0.5
IU required = body weight (kg) x target increase in factor IX (% or IU/dl)
The major complication with these drugs that can occur in patients is the development of antibodies (inhibitors) to the administered factors VIII or IX. This antibody acts by neutralising the administered factor7,12.
Inhibitors (mainly IgG) act directly against the absent factor. The development of inhibitors is more frequent in cases with haemophilia A than in those with haemophilia B. The inhibitors are more likely to develop in cases with severe disease13,14 , astreatment options are limited and mortality is increased. Emicizumab, a bispecific antibody to Factors IXa and X that carries out the function of Factor VIII (FVIII). ITI therapy or bypass is a method of treating patients who develop inhibitors that typically involves administering infusions of large doses of clotting factor concentrates to a patient daily for many weeks or months. The goal of ITI therapy is to stop the inhibitor reaction from occurring in the blood and to condition the body to accept clotting factor concentrate treatments15.
The treatment of haemophilia involves a huge economic cost for health systems. This expense is due to the elevated price of the coagulation factor concentrates that patients need throughout their lives to avoid and/or minimize the complications of the haemorrhages. Therefore, it is extremely important to know the cost of haemophilia treatment, and above all to know if there are ways to reduce this cost without detriment to the quality of treatment16.
The present study aims to estimate the economic burden for the national health system based on the consumption of haemophilic treatments in patients with HA and HB in a first level hospital in the community of Madrid from 2011 to 2020.
Material and methods
A descriptive and comparative analysis of the consumption and cost of haemophilia treatments in the reference hospital of the Community of Madrid during the years 2011 to 2020, the Hospital Universitario de La Paz, has been carried out. The costs that have been included are only factor consumption and other costs have not been taken into consideration (for example: admissions to hospital, absences for work and leave-pays, etc). The data obtained belong to the consumption of each patient treated in the hospital pharmacy. The source of data used was the Dispensing System of the pharmacy of the Hospital Universitario de La Paz in Madrid. The following variables were obtained from this database: number of patients anonymised by history number, age, sex, place of birth, diagnosis (haemophilia A or B), development of inhibitors, type of treatment by national code and trade name, as well as the number of international units (IU) consumed and total expenses.
For this study, patients were stratified by sex, although females were not under the scope of the study, age range (from 0 to 50 every 5 years, 51 to 75 and >75), type of diagnosis (haemophilia A or B), degree of severity and bypass agent use (treatment with Feiba® and/or Novoseven® was analysed).
Data extraction from computerized records was anonymized and did not include variables that could identify patients, as the study focus only on consumption and costs, so the study respects the confidentiality of the data.
The costs were calculated based on the number of IU consumed by patients from the hospital pharmacy, not considering the returns of units not consumed, to have a true representation of the consumption of packages and their cost to the Health System of Madrid. These costs are reflected in net price (€).
Epidemiology of patients
The patients included in the study were those treated at the Hospital Universitario de La Paz, diagnosed of haemophilia A or B, excluding those with Von Willebrand disease and deficiencies of a specific factor. Of the total number of patients included, 701 patients, 85% had haemophilia A and 15% haemophilia B. Of these, 68% and 71% patients respectively had severe haemophilia, 22% and 12% respectively moderate haemophilia, and 11% and 18% respectively mild haemophilia. Patients presenting inhibitors were 1% and 8% respectively (haemophilia A and haemophilia B). Of the total number of patients only males have been included in the study. The median age of all patients was 32.6 with a standard deviation of 21.36.
For the severity, use of bypass agents and age, 2020 has been taken as the reference year and results were based on the patients from that year.
Results
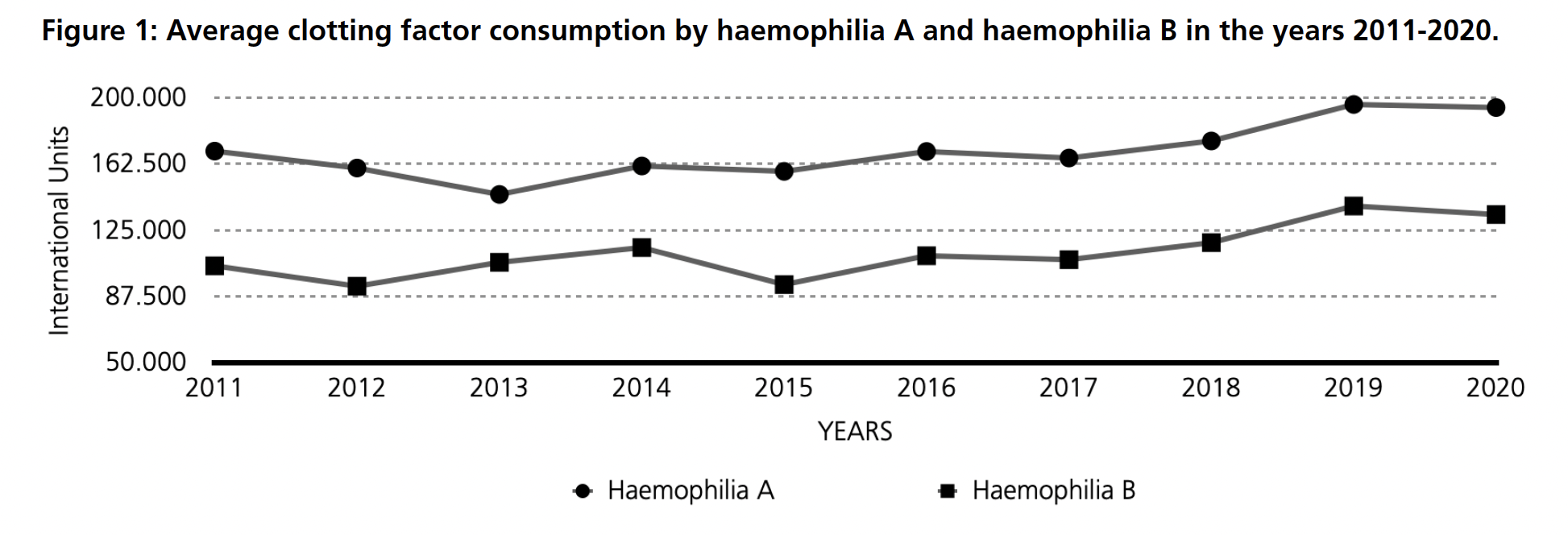
The average consumption per patient of clotting factors used in the treatment of haemophilia A and B at La Paz Hospital in Madrid during the years 2011 to 2020 (Figure 1), is estimated to be between 150,000 and 200,000 IU for haemophilia A and between 100,000 and 150,000 IU for haemophilia B.
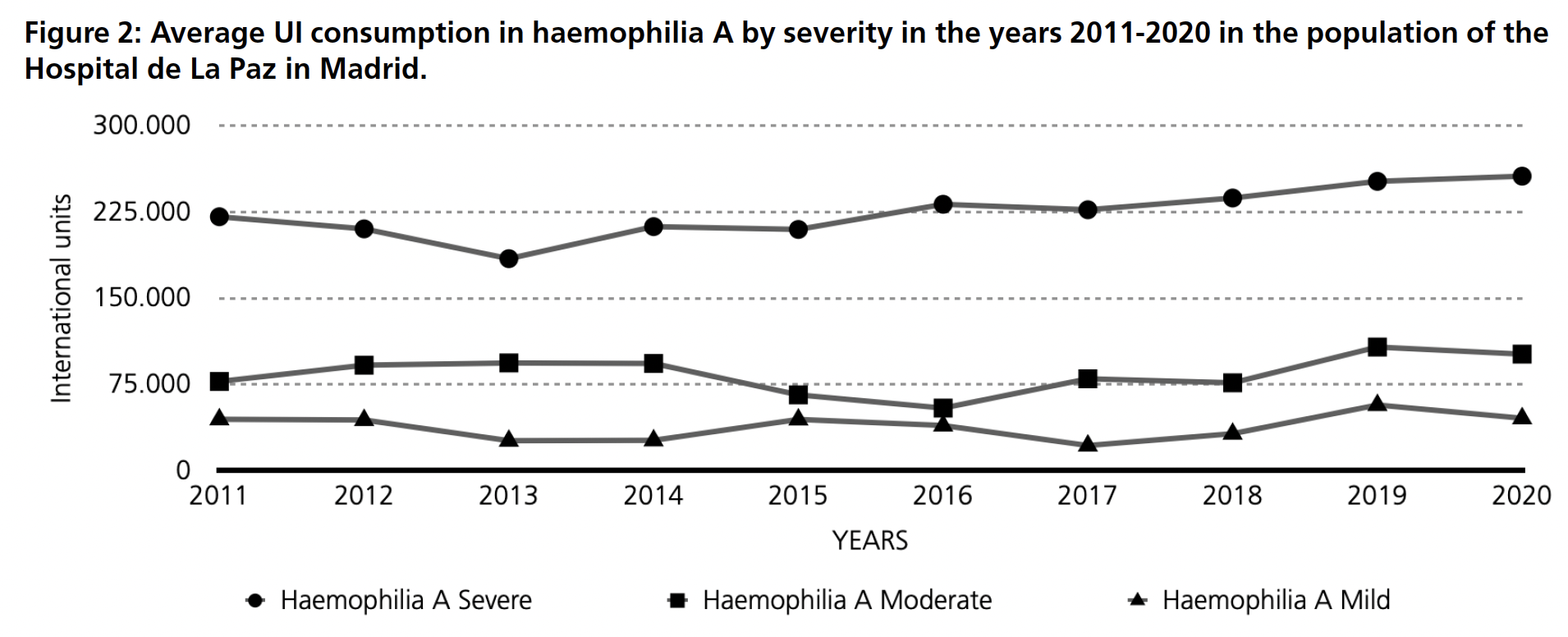
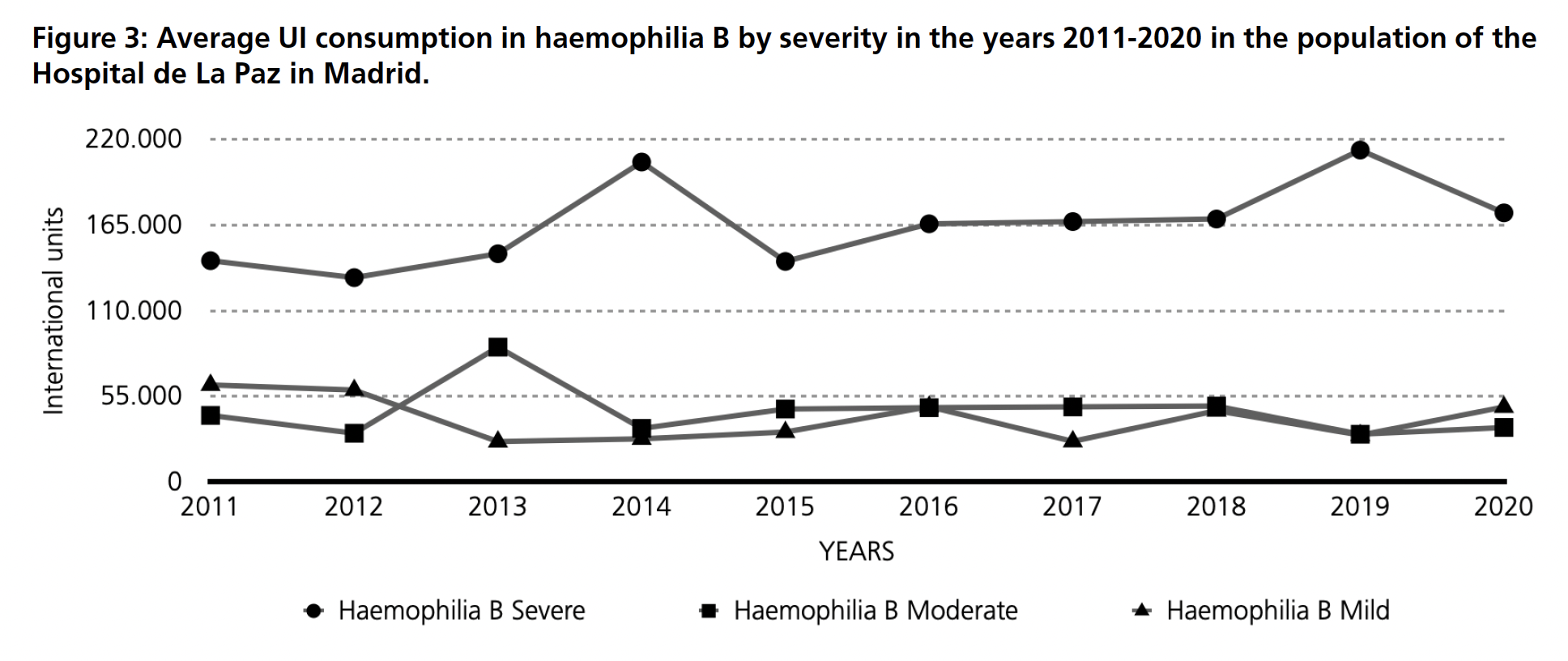
An average annual consumption over the same period was obtained and classified by haemophilia severity. For haemophilia A (Figure 2), the consumption of clotting factors for severe haemophilia ranges between 170,000 and 210,000 IU per patient per year. For patients with moderate and mild haemophilia A, consumption is estimated to be between 50,000 and 100,000 IU for the former and up to 50,000 IU for the latter. For haemophilia B (Figure 3), factor consumption in patients with severe haemophilia B varies between 130,000-210,000 IU per patient. For moderate and mild haemophilia B, the ranges are between 40,000 and 90,000 IU in moderate and 30,000 to 60,000 IU per patient.
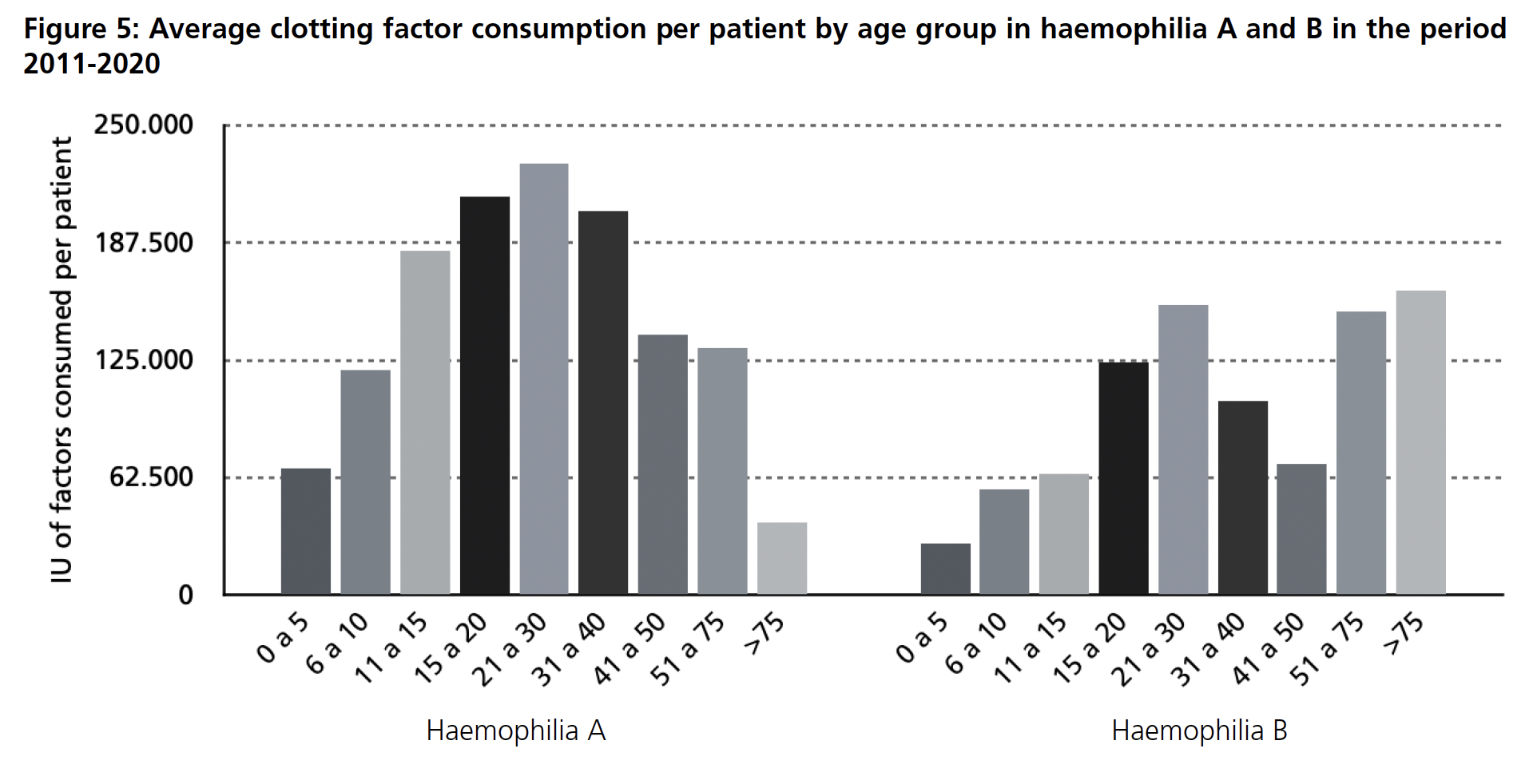
If the average costs on haemophilia A treatment is analysed by age group (Figure 4), it is estimated that patients aged 15-20 years and 21-30 years have the highest cost per patient, around 94,000 euros in the first group and 96,000 euros in the second. The lowest costs are for patients aged 0-5 years and over 75 years, with an approximate cost of €34,000 and €17,000 respectively. For haemophilia B (Figure 4), the highest estimated cost corresponds to the age groups 21-30 years, 51-75 years and over 75 years, between €65,000 and €75,000 per patient. The age group with the lowest expenditure is estimated to be between 0-5 years of age. Together with costs, the average consumption studied (Figure 5) shows that the age group with higher needs of factor are 15-20, 21-30 and 31-40 in haemophilia A while for haemophilia B it is also 21-30 but followed by 51-75 and with the higher demand, over 75 years old.

In the case of patients who have an inhibitor included on their treatment (Feiba® and/or Novoseven®), meaning patients presenting high titre of inhibitors, compared to those patients who do not (Table 1), the average consumption in the former group for both haemophilia A and B is significantly higher than in patients who do not have these inhibitors included in their treatment.

Discussion
The present descriptive observational study analyses the economic impact caused by haemophilia in the treated population of a reference hospital in the Community of Madrid. Few studies have analysed the economic impact of this disease in Spain16. Several European and American studies have been able to verify the effect of the pathology, either by treatment or due to the clinical manifestations presented by these patients, as well as the appearance of inhibitors15,17–19.
Haemophilia A and B and its severity
Haemophilia requires lifelong treatment, consisting not only of treatment of bleeding episodes but also prophylaxis. The prescription and consumption of drugs dedicated to the treatment of patients with both haemophilia and haemophilia B differs, as does the amount of drug to be administered to achieve adequate factor levels in the blood. Depending on the type of haemophilia and the severity of the haemophilia, different drug infusions will be needed. For this, it is important to know the pharmacokinetics of the factor and to focus on a cost-effective strategy8,20. Factor replacement products are among the most expensive therapies, with a total annual cost rising to more than $250,000 per adult patient in the United States. The overall cost per patient with haemophilia and complications associated with the development of inhibitors in this country can be as much as $1 million annually15. A study based on a nationwide cohort in Finland concluded that patients who received factor replacement therapy had a mean total annual cost of treatment ranged from €2520 to €176,330 for both haemophilia A and B18. As per results observed in this study, costs in Spain are like those that were estimated for the US and are also in line with the ones from Finland.
Depending on the severity of the pathology, cost and consumption is associated with bleeding episodes. Patients affected by severe haemophilia A or B present with repeated, spontaneous, and post-traumatic bleeding episodes that seriously interfere with their everyday lives. Recurrent haemarthrosis leads to serious deterioration of joint structures, with a consequent reduction in function and atrophy of the associated skeletal muscles. In the last decade, there has been a continuous improvement in the treatment of patients with haemophilia, thanks to the availability of recombinant concentrates characterised by high efficacy and safety, and to the widespread adoption of prophylaxis as the replacement therapy regimen21.
Patients with severe haemophilia are at greater risk of haemophilia-related complications, including hemarthrosis, haemophilic arthropathy, and development of inhibitor antibodies. As the largest group of the haemophilia population and consisting of those who need the highest level of service, patients with severe disease account for most of the cost of care15. In the study conducted, the percentage of patients with severe haemophilia were 22% and 12% (HA/HB), with moderate haemophilia, and 11% and 18% respectively for mild haemophilia .These data are in line with other studies conducted22.
For haemophilia B, in haemophilic patients, during 2013 it can be seen a significant difference in consumption compared to the previous and upcoming years. Analysing the data, it was seen that during this year, more immunotolerance had been consumed in prophylaxis, meaning that the average of UIs was higher given precautionary measures of prophylaxis. For the second significant difference, in 2019, it was not immunotolerance but higher prophylaxis and UIs on demand were consumed, making the consumption during this year higher than the years before. Additionally for HB, in this case mild and moderate suffering patients, during 2011 mild consumption levels are higher than moderate ones, happening the same during 2012 and 2020, demand and prophylaxis was prescribed at a higher level, for some specific age groups.
Age
Another crucial factor influencing the cost of haemophilia care is age. Some studies show that children with haemophilia use significantly more factors concentrate at a higher cost compared with adult patients. In this study, and in others carried out also in Spain, it is observed that the highest factor cost per treatment is in young adults (from 15 onwards) and adults, and for this study more specifically in the 15-20- and 20-30-years age group15,23. This makes sense as prophylaxis treatment during these ages is higher and intensive while in older ages, they have a more specific and personalized treatment based on their daily activity. It has also been reported that incremental recovery rates with factor replacement treatment are lower for patients 15 years or younger than for those who are older than 15 years. When compared to the neighbouring country, Portugal, annual costs per patient estimated to be €56,875 of which €53,948 (94.9%) correspond to direct costs, which are those analysed in the present study, for haemophilia A. Cost estimates by age group consists of €72,287 per paediatric patient and €51,737 per adult, taking as paediatric population patients under 18 years of age24. It can be significant the difference between expenses and consumption for haemophilia B. The reason for this is that the age group between 21-30 used more prophylactic treatment than the age groups from 31-40 and 41-50, who have higher expenses as per Figure 4, but when analysing which treatments they received, for age groups from 31-40 and 41-50, inhibitors were highly used. This makes the total expense for those age groups higher at a lower UIs consumption due to the price that these treatments have.
Inhibitors
As cited before and in any economic study conducted on the impact of haemophilia, haemophilia patients who develop inhibitors to the clotting factors administered must be considered. The cost of care for patients with high inhibitor titres is generally higher than for patients without inhibitors. In addition, people with haemophilia who develop an inhibitor are twice as likely to be hospitalised because of a bleeding complication. The management of people who develop inhibitors is complex and is one of the biggest challenges in haemophilia care today. The healthcare costs associated with inhibitors can be staggering due to the cost and the amount of factor product needed to stop bleeding15,24. In patients without inhibitors in a Portuguese study, annual cost estimates are 401 euros for mild patients, 5327 euros for moderate patients and 85,805 euros for severe patients. The average annual cost per patient without inhibitors is 39,654 euros and 302,189 euros per patient presenting inhibitors. Approximately 0.3% of haemophilia A expenditure is associated with mild cases without inhibitors (37.5% of all patients), 1.5% with moderate cases without inhibitors (14.2% of all patients), 63.4% with severe cases without inhibitors (41.9% of all patients) and 34.9% with patients with inhibitors (6.5% of all patients). In other studies, conducted in Spain, the average annual cost of a haemophilia A patient with inhibitors in Spain was estimated at €430,227, with the cost of the prophylactic strategy being €636,905 per adult patient and €264,819 per paediatric patient23 These results are consistent with previous cost burden analyses performed for both haemophilia A and B and with the results obtained in this study25.
Conclusion
Haemophilia is a pathology with a great social and economic-health impact. This is not only due to the high mortality rate but also to the complications caused by the development of inhibitors in these patients. The costs of haemophilia are mainly due to the clinical manifestations of the disease 2,15. Despite being rare, haemophilia remains one of the most costly and challenging diseases to manage in every country. This study allowed to have a picture of the impact of haemophilia A and B in a 10-year time analysis. It has been seen that age, severity, and presence of inhibitors have a different impact in cost and consumption of factors. Severity and inhibitors are clearly the two factors that have the higher impact for the National Health System, not only for Spain but for the other countries too.
Limitations
This study has several limitations as only consumption of haemophilic factors, without considering hospitalisations or other resources (caregivers, work-absences or disability), as well as the pharmaceutical costs of a single hospital have been taken into account. Furthermore, it does not consider whether they are due to one-off bleeding episodes, or they reflect ongoing treatment of the population. Despite this, it is estimated that it may reflect the consumption and cost of this pathology and its impact on the Spanish healthcare system. Another limitation is that the study focuses exclusively on patients with haemophilia and does not consider the costs for other coagulation pathologies or the use for other reasons. Moreover, the analysis does not further differentiate between on-demand and prophylaxis treatment, as all of them are included as a bulk.
The authors declare no conflict of interest
Acknowledgements: No financial support has been received for this article.
Data support is to be thanked to our dear co-author Jose Antonio Romero.
Bibliografía
- Mannucci PM. Hemophilia therapy: the future has begun. Haematologica [Internet]. 1 de marzo de 2020 [citado 15 de marzo de 2022];105(3):545-53. Disponible en: https://haematologica.org/article/view/9274
- García-Sacristán A, Domínguez-Pinilla N, Cuesta-Tovar J, García-Palomo M. García-Sacristán, AA, Domínguez-Pinilla, N, Cuesta-Impacto económico de emicizumab en pacientes con hemofilia a con inhibidor en un hospital de tercer nivel. Rev OFIL [Internet]. 16 de agosto de 2021 [citado 3 de septiembre de 2022];31(2):167-71. Disponible en: https://dx.doi.org/10.4321/s1699-714×2021000200010
- Sidonio RF, Malec L. Hemophilia B (Factor IX Deficiency). Hematol Oncol Clin North Am [Internet]. 1 de diciembre de 2021 [citado 3 de septiembre de 2022];35(6):1143-55. Disponible en: https://www.sciencedirect.com/science/article/pii/S0889858821000873
- Agencia Española de Medicamento y Productos Sanitarios (AEMPS). Informe de Posicionamiento Terapéutico de emicizumab (Hemlibra®) en hemofilia A [Internet]. 2020 [citado 27 de septiembre de 2022]. Disponible en: https://www.aemps.gob.es/informa/informes-de-posicionamiento-terapeutico/informe-de-posicionamiento-terapeutico-de-emicizumab-hemlibra-en-hemofilia-a/
- García-Escribano N. Informe de Posicionamiento Terapéutico de albutrepenonacog alfa (Idelvion®) en hemofilia B. :7.
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia [Internet]. enero de 2013 [citado 16 de enero de 2022];19(1):e1-47. Disponible en: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2516.2012.02909.x
- L’hémophilie [Internet]. MHEMO. [citado 16 de enero de 2022]. Disponible en: https://mhemo.fr/les-pathologies/lhemophilie/
- Páramo JA. Treatment of haemophilia: From replacement to gene therapy. Med Clin (Barc). 24 de diciembre de 2021;157(12):583-7.
- White GC, Kempton CL, Grimsley A, Nielsen B, Roberts HR. Cellular immune responses in hemophilia: why do inhibitors develop in some, but not all hemophiliacs? J Thromb Haemost JTH. agosto de 2005;3(8):1676-81.
- Ar MC, Balkan C, Kavaklı K. Extended Half-Life Coagulation Factors: A New Era in the Management of Hemophilia Patients. Turk J Hematol [Internet]. septiembre de 2019 [citado 6 de noviembre de 2022];36(3):141-54. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6682782/
- EMA. Advate [Internet]. European Medicines Agency. 2018 [citado 10 de febrero de 2022]. Disponible en: https://www.ema.europa.eu/en/medicines/human/EPAR/advate
- Teitel JM. Treatment and prevention of bleeding in congenital hemophilia A patients with inhibitors. Transfus Apher Sci [Internet]. 1 de agosto de 2018 [citado 3 de septiembre de 2022];57(4):466-71. Disponible en: https://www.trasci.com/article/S1473-0502(18)30300-8/fulltext
- Langer AL, Etra A, Aledort L. Evaluating the safety of emicizumab in patients with hemophilia A. Expert Opin Drug Saf. diciembre de 2018;17(12):1233-7.
- Malkan UY, Aksu S. Combination of Novoseven and Feiba in Hemophiliac Patients with Inhibitors. Open Med [Internet]. 24 de diciembre de 2018 [citado 3 de septiembre de 2022];13:618-21. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6310920/
- Dan R. Dalton MS. Hemophilia in the Managed Care Setting. Suppl Featur Publ [Internet]. 1 de abril de 2015 [citado 4 de septiembre de 2022];21(6 Suppl). Disponible en: https://www.ajmc.com/view/ace0024_mar15_hemophilia_dalton
- Rodriguez-Merchan EC. The cost of hemophilia treatment: the importance of minimizing it without detriment to its quality. Expert Rev Hematol. marzo de 2020;13(3):269-74.
- Bolous NS, Chen Y, Wang H, Davidoff AM, Devidas M, Jacobs TW, et al. The cost-effectiveness of gene therapy for severe hemophilia B: a microsimulation study from the United States perspective. Blood [Internet]. 4 de noviembre de 2021 [citado 24 de septiembre de 2022];138(18):1677-90. Disponible en: https://doi.org/10.1182/blood.2021010864
- Characterisation of healthcare utilisation and cost of haemophilia care in real‐life: A 4‐year follow‐up study in Finland – Ventola – 2021 – Haemophilia – Wiley Online Library [Internet]. [citado 28 de septiembre de 2022]. Disponible en: https://onlinelibrary.wiley.com/doi/10.1111/hae.14197
- Cowie MR, Lamy A, Levy P, Mealing S, Millier A, Mernagh P, et al. Health economic evaluation of rivaroxaban in the treatment of patients with chronic coronary artery disease or peripheral artery disease. Cardiovasc Res. 1 de septiembre de 2020;116(11):1918-24.
- Villarrubia R, Oyagüez I, Álvarez-Román MT, Mingot-Castellano ME, Parra R, Casado MA. Cost analysis of prophylaxis with activated prothrombin complex concentrate vs. on-demand therapy with activated factor VII in severe haemophilia A patients with inhibitors, in Spain. Haemophilia [Internet]. 2015 [citado 24 de septiembre de 2022];21(3):320-9. Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1111/hae.12681
- Cortesi PA, D’Angiolella LS, Lafranconi A, Micale M, Cesana G, Mantovani LG. Modern Treatments of Haemophilia: Review of Cost-Effectiveness Analyses and Future Directions. PharmacoEconomics [Internet]. 1 de marzo de 2018 [citado 24 de septiembre de 2022];36(3):263-84. Disponible en: https://doi.org/10.1007/s40273-017-0588-z
- Aznar JA, Lucía F, Abad-Franch L, Jiménez-Yuste V, Pérez R, Batlle J, et al. Haemophilia in Spain. Haemophilia [Internet]. 2009 [citado 10 de diciembre de 2021];15(3):665-75. Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2516.2009.02001.x
- Jiménez-Yuste V, Álvarez Román MT, Mingot-Castellano ME, Fernández Mosteirin N, Mareque M, Oyagüez I. Análisis de costes del tratamiento para pacientes con hemofilia A con inhibidor en España. PharmacoEconomics Span Res Artic [Internet]. 1 de agosto de 2018 [citado 4 de septiembre de 2022];15(1):25-34. Disponible en: https://doi.org/10.1007/s40277-018-0080-y
- Café A, Carvalho M, Crato M, Faria M, Kjollerstrom P, Oliveira C, et al. Haemophilia A: health and economic burden of a rare disease in Portugal. Orphanet J Rare Dis [Internet]. 4 de septiembre de 2019 [citado 28 de septiembre de 2022];14(1):211. Disponible en: https://doi.org/10.1186/s13023-019-1175-5
- Li N, Sawyer EK, Maruszczyk K, Guzauskas G, Slomka MT, Burke T, et al. Adult lifetime cost of hemophilia B management in the US: payer and societal perspectives from a decision analytic model. J Med Econ [Internet]. 1 de enero de 2021 [citado 26 de septiembre de 2022];24(1):363-72. Disponible en: https://www.tandfonline.com/doi/full/10.1080/13696998.2021.1891088
____
