Guzmán-Ramos MI1, Díaz-Acedo R1, Galván-Banqueri M1, Saborido-Cansino C2, Gutiérrez-Pizarraya A1, Márquez-Saavedra E1
1 UGC Farmacia. Hospital Universitario Virgen de Valme. Sevilla (Spain)
2 Área de Gestion Sanitaria Sur. Sevilla (Spain)
Fecha de recepción: 01/05/2020 – Fecha de aceptación: 10/06/2020
Correspondencia: Antonio Gutiérrez-Pizarraya – Pharmacy Unit. Hospital Universitario Virgen Valme – Ctra. de Cádiz Km. 548,9 – 41014 Seville (Spain)
boticariors@gmail.com
____
SUMMARY
Objectives: Sacubitril/valsartan is a drug for chronic heart failure (CHF), approved by Drugs Regulatory Agencies based on the results of the PARADIGM-HF, which could have several limitations on internal validity and applicability. Furthermore, this drug has a high economic impact.
The objectives of this study are to evaluate effectiveness and safety of sacubitril/valsartan in CHF, as well as to evaluate adequation to use criteria stablished in a Health Management Area (HMA).
Methods: Retrospective, observational study including adult patients with CHF who were receiving sacubitril/valsartan during 2017 in an HMA. The treatment effectiveness was assesed by death and/or hospitalization rates related to CHF. Frequency of adverse events was used to safety evaluation. Furthermore, adequation rate was assessed.
Findings: A total of 68 patients were included. Death or hospitalization rates due to CHF at 12 months were 32.3% globally (2.9% and 29.4% respectively). Among patients analyzed, 33.8% presented hypotension, during the first year after treatment initiation. Overall adequation rate was 67.6%.
Conclusions: A high percentage of death and/or hospitalization due to CHF was observed. Hypotension is a frequent adverse event which leads to dose adjustment and/or drug withdrawal. Overall adequation rate of sacubitril/valsartan prescription is acceptable.
Key words: Chronic heart failure, sacubitril/valsartan, hypertension, pharmacotherapy, real-life study.
Estudio en vida real de la terapia combinada de sacubitril valsartán en insuficiencia cardíaca crónica
RESUMEN
Objetivos: El sacubitril/valsartán es un medicamento para la insuficiencia cardíaca crónica (ICC), aprobado por las agencias reguladoras de medicamentos en base a los resultados del ensayo pivotal PARADIGM-HF, que podría tener varias limitaciones en la validez interna y la aplicabilidad. Además, este fármaco tiene un alto impacto económico. Los objetivos de este estudio son evaluar la efectividad y la seguridad de sacubitril/valsartán en la ICC, así como evaluar la adecuación a los criterios establecidos en un Área de Gestión de Salud (AGS).
Métodos: Estudio observacional retrospectivo que incluye pacientes adultos con ICC que recibieron sacubitril/valsartán durante 2017 en una AGS. La efectividad del tratamiento fue evaluada mediante la tasa de mortalidad y/o hospitalización relacionadas con la ICC. La frecuencia de los eventos adversos se utilizó para la evaluación de seguridad. Además, se evaluó la tasa de adecuación.
Resultados: Se incluyeron un total de 68 pacientes. Las tasas de mortalidad u hospitalización por ICC a los 12 meses fueron del 32,3% a nivel global (2,9% y 29,4%, respectivamente). Entre los pacientes analizados, el 33,8% presentó hipotensión durante el primer año después del inicio del tratamiento. La tasa de adaptación global fue del 67,6%.
Conclusiones: Se observó un alto porcentaje de muerte y/o hospitalización por ICC. La hipotensión es un evento adverso frecuente que conduce al ajuste de la dosis y/o a la retirada del medicamento. La tasa general de adecuación de la prescripción de sacubitril/valsartán es aceptable.
Palabras clave: Insuficiencia cardíaca crónica, sacubitril/valsartán, hipertensión, farmacoterapia, estudio en vida real.
____
INTRODUCTION
Chronic heart failure (CHF) is a condition which leads to frequent hospitalizations, quality of life deterioration and high rates of mortality. Pharmacological treatment in CHF focuses on relieving symptoms (such as dyspnea or fluid retention), preventing readmissions and improving survival1.
The recommended CHF treatment combines renin-angiotensin-aldosterone system inhibitors, such as angiotensin-converting enzyme inhibitor (ACEI) or angiotensin-II receptor blockers (ARB), and beta-blockers. In refractory cases, the addition of aldosterone antagonists, such as spironolactone, is proposed. To relieve congestive symptoms, diuretics are used. This is reflected in the treatment algorithms of European1 and American Cardiology Societies2 .
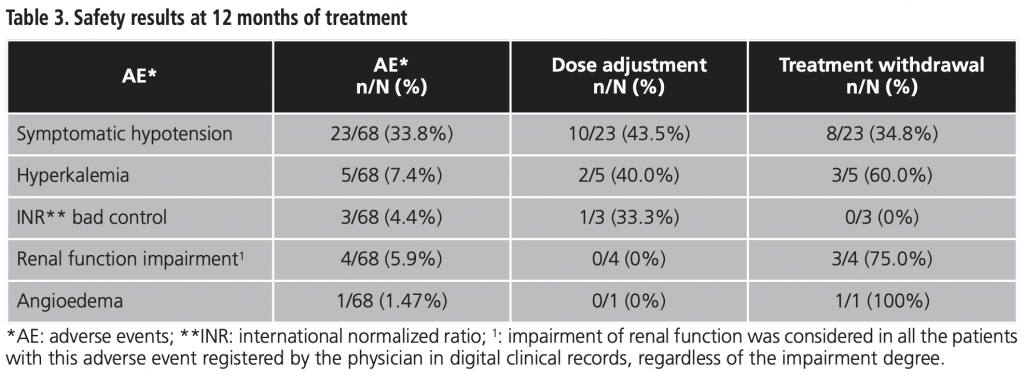
Sacubitril/valsartan is a new drug for CHF treatment which inhibits neprilysin and blocks angiotensin-II type-I receptor. The Spanish Agency of Medicines and Health Products (AEMPs) published an assessment report (therapeutic positioning report) for sacubitril/valsartan3 on the basis of which, the Commission for the Rational Use of Medicines of the South of Seville Health Management Area (HMA) stablished some criteria for prescribing this drug. These criteria are: left ventricular ejection fraction (LVEF) <35%, New York Heart Association (NYHA) class II-III, and finally ACEI or ARB treatment during the last 4 weeks before starting sacubitril/valsartan without achieving adequate control of CHF symptoms.
The pivotal clinical trial (RCT)4 was a randomized, controlled and double-blind, multicenter trial comparing sacubitril/valsartan and enalapril. It included 8,442 patients with CHF with NYHA class II-IV and LVEF ≤40% (subsequently modified to ≤35%) on previous treatment with ACEI or ARB. The main combined variable was death and/or hospitalization due to CHF. The trial was stopped early (at 27 months of follow-up) because sacubitril/valsartan achieved a reduction of 4.7% for the combined end of death and/or hospitalization, reaching statistical significance (HR 0.8, 95% CI 0.73-0.87, p<0.001), as well as a significantly reduction of crude mortality (HR 0.84; 95% confidence interval 0.76-0.93, p<0.001).
However, this RCT presents some limitations of internal validity (unbalanced baseline characteristics between arms) and applicability (sacubitril/valsartan dose poorly tolerated in clinical practice and tolerant population selected by a run-in phase) which introduce a reasonable uncertainty about its reproducibility in real populations5. In addition, the economic impact of this treatment is high. Because of that, it is necessary to identify patients with the most favorable benefit/risk balance6.
The objective of this study is to evaluate the effectiveness and safety of sacubitril/valsartan for CHF treatment, as well as the adequation to the criteria of use recommended in the South of Seville HMA.
METHODS
This is an observational, retrospective study conducted in an HMA covering aproximately to 406,768 inhabitants. All patients older than 18 years with a diagnosis of CHF and undergoing treatment with sacubitril/valsartan between January-December 2017 were included. As exclusion criteria, previous or current participation in RCTs were considered.
Demographic (sex and age) and diagnostic variables (prescription physician, NYHA class and LVEF at the beginning of sacubitril/valsartan treatment and ACEI/ARB previous treatment) were recorded.
Mortality and/or hospitalization due to CHF at 12 months was stablished as main effectiveness variable. Some secondary variables were also defined: blood pressure (BP) control, symptomatic improvement of CHF at 12 months and NYHA class variation at 12 months.
To assess safety, adverse events (AE) and dose adjustment and/or treatment withdrawal during the study period was considered.
Overall adequation rate for sacubitril/valsartan treatment was evaluated by the percentage of compliance with these three items: LVEF ≤35%, NYHA class I-II and previous ACEI/ARB treatment.
Patients with an active sacubitril/valsartan prescription were obtained from the electronic prescription application available in the Andalusian Health Service. Data were collected from digital medical records. SPSS.v.21® was used for statistical analysis.
This study was approved by the Ethics Committee of the South of Seville HMA.
RESULTS
A total of 68 patients who met the inclusion criteria were recruited, whit a mean age of 68.4 years. Of them, 69.1% were men, mainly receiving therapy prescribed by cardiology department (70.6%). The rest of baseline features are shown in table 1.
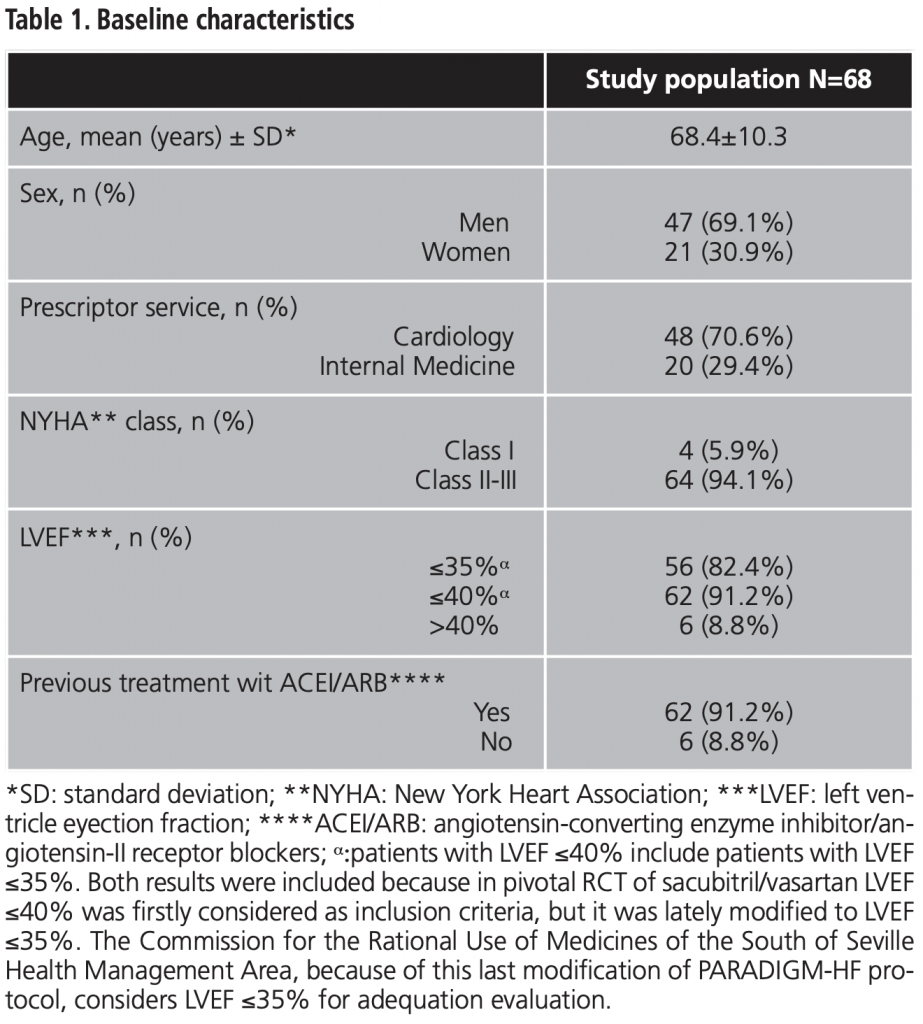
After 12 months of treatment, 22 patients (32.4%) had died and/or had been hospitalized due to CHF. All the effectiveness results at 12 months are shown in table 2.
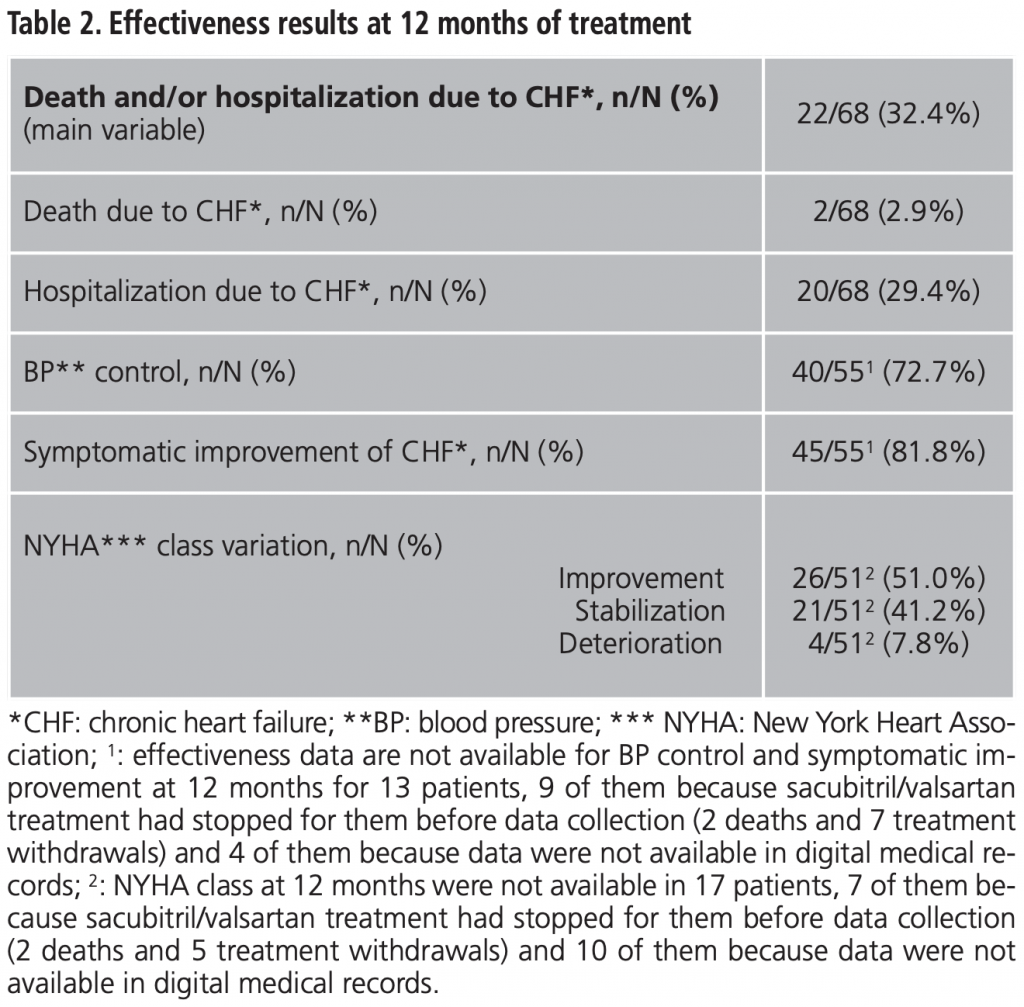
Adverse events observed during first 12 months of treatment, as well as need for dose adjustment and/or treatment withdrawal rates are shown in table 3. Hypotension was the most frequent AE (33.8% of patients, n=23).
Overall adequation rate was achieved in 46 patients (67.6%). Moreover, adequation for each individual item was as follow: LVEF ≤35% in 56 patients (82.4%), New York Heart Association (NYHA) II-III in 64 (94.1%) and 62 with previous angiotensin-converting enzyme inhibitor/angiotensin-II receptor blockers (ACEI/ARB) treatment (91.2%).
Since the RCT PARADIGM-HF presents several limitations5 and sacubitril/valsartan has an important economic impact in health services6, it is important to evaluate these real-life results. So far, this work is the first study which evaluates effectiveness and safety of sacubitril/valsartan based on the same variables which were stablished in the pivotal RCT4.
In our study, patients had a mean age higher than PARADIGM-HF patients (68.4 years vs. 63.8) and a proportion of men lower (69.1% vs. 78.2). The European Public Assessment Report of sacubitril/valsartan shows a value for the combined variable of death and/or hospitalization due to CHF at 12 months lower than 12%7 (extracted from PARADIGM-HF data), which is lower than ours (32.4%). This difference could be due to the differences in demographic patient’s characteristics.
Higher prevalence of hypotension in real life is remarkable (33.8% after 12 months vs. 17.6 at month 27), and it is probably due to the run-in phase performed in the pivotal RCT4. Hypotension could be an important limitation in clinical practice. Thus, the rate of treatment withdrawal because of hypotension was 11.7% in our study vs. 0-9% in PARADIGM-HF4. Other treatments withdrawal rates in our population (compared to pivotal RCT results)4 were: 4.4% vs. 0.7 for acute renal failure and 4.4% vs. 0.3 for hyperkalemia.
Overall treatment adequation rate was acceptable (67.6%). The main reason for non-adequacy was a LVEF >35% in 17.6% of patients, but only an 8.8% of patients had a LVEF >40%.
In 2017, the Catalan Health Service published a retrospective study, including 2,179 patients and describing sacubitril/valsartan use. The 75% of patients were men (compared to 69.1% in our study) and the mean age of patients was similar than ours (69.8 vs. 68.4 years). All patients, except one, had previously received ACEI/ARB treatment and, in most cases, the lowest dose of sacubitril/valsartan was prescribed, probably due to concerns about hypotension in elderly population8. Moreover, a recent cohort study from the German IMS® prescription database showed that 2/3 of patients with sacubitril/valsartan was receiving low doses (50 mg/12 hours) and only 41% of patients were able to be dose-escalated9. This trend to maintain low doses could be an indicator of higher incidence of hypotension in real clinical practice.
Another recent observational study10 included 201 patients with the same inclusion criteria (NYHA ≥II, LVEF ≤35%, previous ACEI/ARB) which were followed during a mean (±SD) of 221±11.4 days. The proportion of men was higher than ours (82.0% vs. 69.1), while the mean age is similar (67.7 vs. 68.4 years). Regarding the variation in NYHA class at the end of the follow-up period (compared to our study), 32% vs. 51.0 of patients had improved, 64% vs. 41.2 remained stable and 4% vs. 7.8 had worsened.
The main limitation of this study is the inclusion of a local population; thus, results might not be representative of other different populations. Furthermore, its retrospective design and the limited follow-up period are also limitations. However, these results could be useful for demonstrating the effectiveness and safety of sacubitril/valsartan in real life clinical practice, considering the same variables as pivotal RCT.
CONCLUSION
In our study, the percentage of mortality and/or hospitalization due to CHF during the first year of sacubitril/valsartan treatment is high. In addition, hypotension is a frequent adverse event, hypotension is a frequent adverse event, which forces extreme precautions in its use and even to withdraw it occasionally. The adequation of sacubitril/valsartan prescription in the South of Seville HMA is acceptable.
Funding: The present investigation has not received specific aid from agencies from the public sector, commercial sector or non-profit entities.
Conflicts of interest: The authors declare no conflicts of interest.
BIBLIOGRAPHY
1. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787-847. doi: 10.1093/eurheartj/ehs104.
2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA Guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guideline. Circulation. 2013;128:e240-e327. doi: 10.1161/CIR.0b013e31829e8776.
3. Informe de Posicionamiento Terapéutico de sacubitrilo/valsartán (Entresto®) en el tratamiento de la insuficiencia cardiaca crónica sintomática en pacientes adultos con fracción de eyección reducida. Madrid (España): Agencia Española de Medicamentos y Productos Sanitarios 2016. https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-sacubitrilo-valsartan-Entresto-insufi_cardiaca.pdf. (Accessed October 30, 2019. 30/10/2019).
4. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. doi: 10.1056/NEJMoa1409077.
5. Ahn R, Prasad V. Do Limitations in the Design of PARADIGM-HF Justify the Slow Real World Uptake of Sacubitril/Valsartan (Entresto)? Cardiovasc Drugs Ther. 2018;32:633-635. doi: 10.1007/s10557-018-6830-x.
6. Sacubitrilo valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. National Institute for Clinical Excellence. Published: 27/04/2016. https://www.nice.org.uk/guidance/ta388/resources/sacubitrilo-valsartan-for-treating-symptomatic-chronic-heart-failure-with-reduced-ejection-fraction-82602856425157.
7. Committee for Medicinal Products for human use (European Medicines Agency). European Public Assessment Report (EPAR): Entresto. European Medicines Agency. Fecha de publicación: https://www.ema.europa.eu/en/documents/assessment-report/entresto-epar-public-assessment-report_en.pdf. Accessed October 30, 2019.
8. Molina A, Vicente M, Gasol M, Carbonell P, López P, Pontes C. A Drug Utilization Study of Sacubitril/Valsartan in Catalonia. Área del Medicamento, Servicio Catalán de Salud, Barcelona, Spain. Rev Esp Cardiol. 2018. doi: 10.1016/j. rec.2018.08.004.
9. Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, et al. Real-world treatment patterns of sacubitril/valsartan: A longitudinal cohort study in Germany. Eur J Heart Fail. 2019;21:588-97. doi:10.1002/ejhf.1465.
10. Lau CW, Martens P, Lambeets S, Dupont M, Mullens W. Effects of sacubitril/valsartan on functional status and exercise capacity in real-world patients. Acta Cardiol. 2018:1-8. doi: 10.1080/00015385.2018.1521054.
____
