Leganés Ramos A, Sanz Márquez S, Herrero Domínguez-Berrueta MC, Hernández Sánchez MV, Pérez Encinas M
Hospital Universitario Fundación Alcorcón. Madrid (España)
____
Rev. OFIL 2016, 26;4:258-263
Fecha de recepción: 21/03/2016 – Fecha de aceptación: 22/04/2016
____
Correspondencia:
Alejandro Leganés Ramos
Hospital Universitario Fundación Alcorcón
C/Budapest, 1
28922 Alcorcón (Madrid)
Correo electrónico: alexlegaram@hotmail.com
____
SUMMARY
Objetive: To describe and analyze the suspected Pulmonary Embolisms (PEs) as adverse drug reaction (ADR) attributed to the use of Hormonal Contraceptives (HCs), identified in the Hospital Pharmacy Unit of a second level hospital, regarding the informative note issued by the AEMPS.
Methods: A retrospective observational study analyzing suspected PE in women using HCs wich were detected in the Hospital Pharmacy Unit through the pharmaceutical validation of treatment for hospitalized patients in a hospital during a two-year period. The cause-effect relationship between drug administration and the ADR is classified according to the Karch-Lasagna algorithm.
Results: Eight cases of PE were detected in the Hospital Pharmacy Unit, in women using HCs, with a mean age of 22.9 years (SD: 2.67) and a mean Risk Factor (RF) of 2.3 (SD: 0.9) per patient, the most frequent being smoking and obesity. In none of the eight cases described, the HC involved was second generation.
Conclusions: HCs are drugs which have demonstrated having a higher benefit than the risks associated with their use. However, it is necessary to assess each patient’s RFs at initiation of treatment with HCs and during said treatment, in order to select the most adequate HC and reduce the risk of PE. It is also important to conduct an adequate healthcare education for patients, which will help to detect and prevent RFs, as well as to identify the signs and symptoms of PE.
Key Words: Pulmonary embolism, venous thrombosis, contraceptive agents.
____
INTRODUCTION
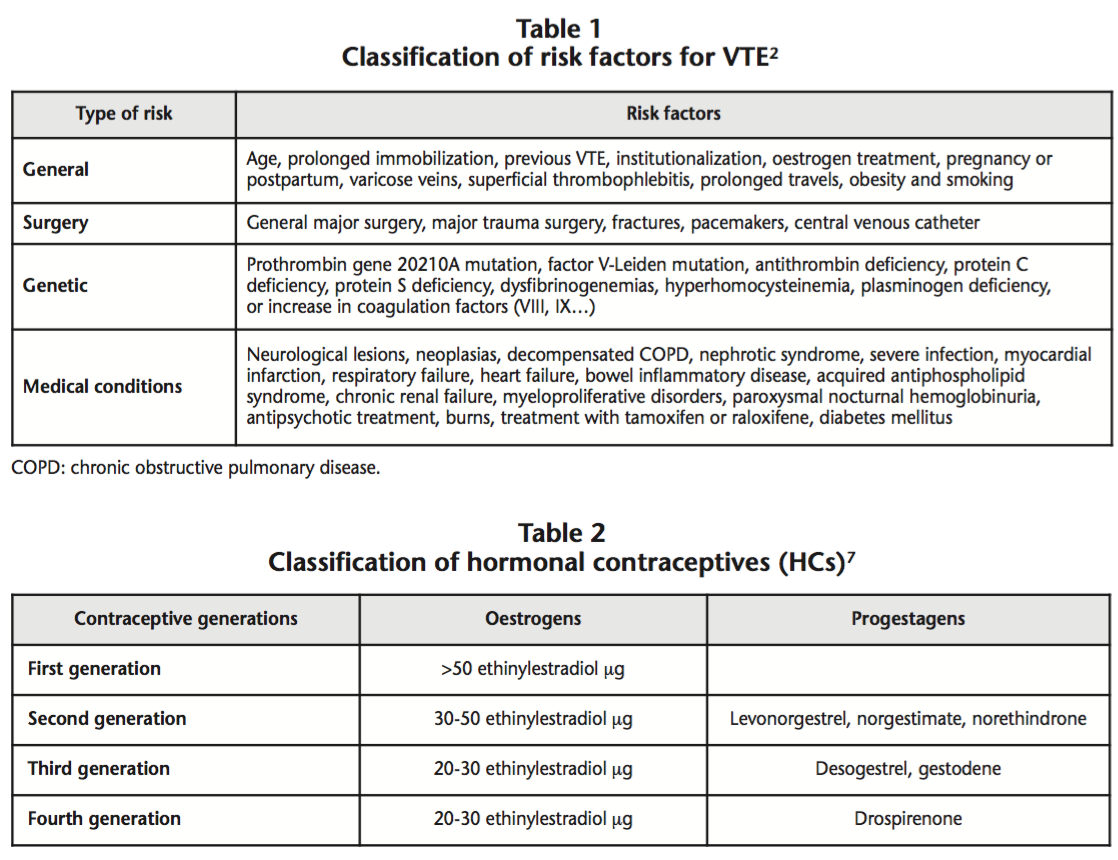
Venous Thromboembolism (VTE) is a clinical term which includes two closely associated conditions: Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE)1,2. There are various risk factors (RFs) associated with an increase in the incidence of PE, and this will be higher when there is a higher number of concomitant RFs3-5. Table 1 shows a classification of RFs for VTE based on their etiology2.
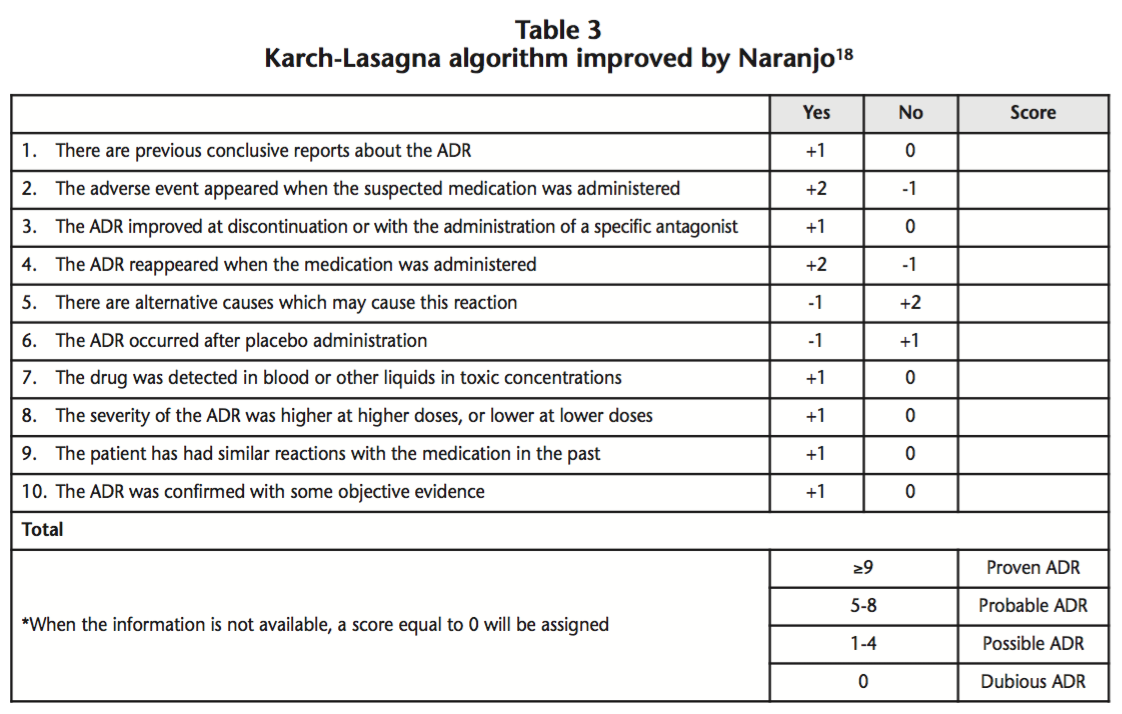
Hormonal contraceptives (HCs) are indicated to prevent pregnancy through a pharmacological method, and they contain combined oestrogens and progestagens. HCs with oral, vaginal or transdermal administration present a high contraceptive efficacy, and an adequate safety profile6. HCs can be classified into different generations, based on the quantity of oestrogens and the type of progestagen they contain (Table 2)7.
First generation HCs are associated with many adverse effects, such as vascular disorders. Second generation HCs cause fewer side effects, though they are also associated with an increased risk of VTE (5-7 cases per each 10,000 woman-years)8. Third generation HCs present a better lipid profile and lower risk of acute myocardial infarction9 than second generation HCs, though the risk of VTE is higher10,11 (6-12 cases per each 10,000 woman-years). Finally, fourth generation HCs present a risk of VTE similar to third generation HCs (9-12 cases per each 10,000 woman-years)8.
HCs containing cyproterone (derived of progesterone) have a high antiandrogenic potency, and a risk of VTE similar to the rest of HCs12. These are only indicated for the treatment of acne, androgenic alopecia, and mild forms of hirsutism, and their use is contraindicated for contraception only. Likewise, chlormadinone is also a derivative of progesterone with a marked antiandrogenic activity13.
The risk of thrombosis associated to the use of HCs seems to be linked with the concentration of oestrogen, with the androgenic potency of the gestagen associated, and the thrombophilic burden of patients, because it has been observed that the pharmacological modulation of oestrogens causes an increase in the concentrations of coagulation factors, and a reduction of the factors inhibiting coagulation9, and these alterations will be higher with third and fourth generation HCs14.
On January, 2013, the Spanish Agency of Medicines and Medical Devices (AEMPS) informed about the initiation of a review of third and fourth generation HCs by the Pharmacovigilance Risk Assessment Committee (PRAC) to assess if the information provided in product specifications and leaflets was enough to make the best treatment decision by healthcare professionals and patients15. Medications containing cyproterone were also evaluated by the PRAC from January, 201316. These reviews ended on October, 2013, and May, 2013, respectively, and the conclusion was that the benefit of using HCs is higher than its risks, though there are slight differences among the different combinations of HCs; the likelihood of VTE is higher during the first year, at treatment re-initiation after an interruption of at least 4 weeks, and in women who present concomitant RFs8. Medications containing cyproterone in combination with ethinyl estradiol are exclusively indicated for the treatment of women with hirsutism and/or androgen dependent acne moderate or severe and/or hirsutism, which do not respond to topical treatment and systemic antibiotic therapy. They should not be taken with other hormonal contraceptives as this would increase the risk of VTE17.
The objective of the present study is to describe and analyze the suspected PEs as adverse drug reaction (ADR), attributed to the use of HCs identified in the Hospital Pharmacy Unit of a second level hospital during a two-year period, regarding the informative note issued by the AEMPS.
MATERIAL AND METHODS
An observational retrospective study, analyzing the suspected PEs secondary to the use of HCs in a second-level hospital from the Community of Madrid, during the period between July, 2012 and July, 2014.
Suspected PEs associated with the use of HCs were detected in the Hospital Pharmacy through the pharmaceutical validation of treatment for hospitalized patients, and were recorded in the Pharmacy Unit database. The electronic clinical record application (Selene®) was used for clinical data collection, as well as the Primary Care prescription viewer (HORUS®) and the manager of clinical requests available at the hospital (GPC®).
The following variables were collected:
– Demographic variables: Age, HC involved, and in case of using cyproterone, its indication.
– Concomitant RFs: Smoking, lack of mobility in the four weeks before the PE, previous VTE, obesity, family history of VTE, and results of thrombophilia testing.
– PE symptoms and signs at admission: Pleuritic pain, dyspnea, haemoptysis, pain or inflammation in lower limbs, tachycardia (>100 beats per minute), tachypnea (>20 breaths per minute) and desaturation (Sat. O2 <95%).
– Lab test data: Partial pressure of oxygen in arterial blood and D-dimer value.
– Diagnostic tests: Electrocardiogram, chest X-ray, computed tomography angiography (CTA), pulmonary scan, transthoracic ultrasound test, and eco-doppler.
– Clinical management: Hospitalization or not in the Intensive Care Unit (ICU), treatment of the acute stage, treatment at hospital discharge, contraindication for HC and other measures and recommendations.
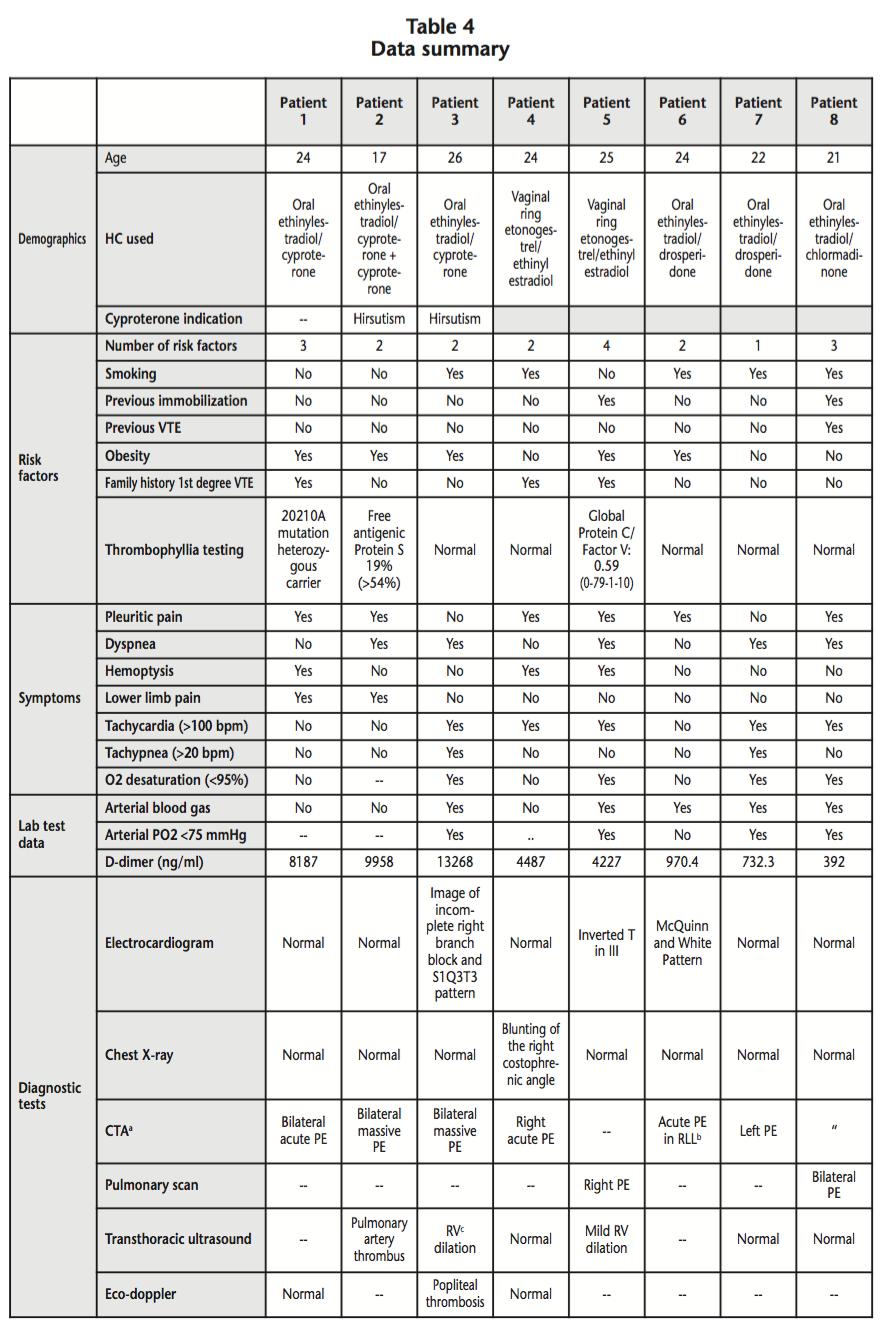
The cause-effect relationship between drug administration and the ADR is classified according to the Karch-Lasagna criteria18, from dubious to proven, as appears in table 3.
All suspected PEs associated to the use of HC were notified with the on-line Yellow Card system to the Pharmacovigilance Centre (PC) of the Autonomous Community of Madrid. The PVS was consulted about the number of suspected PEs associated to the use of HC during the same period of the study at national level.
RESULTS
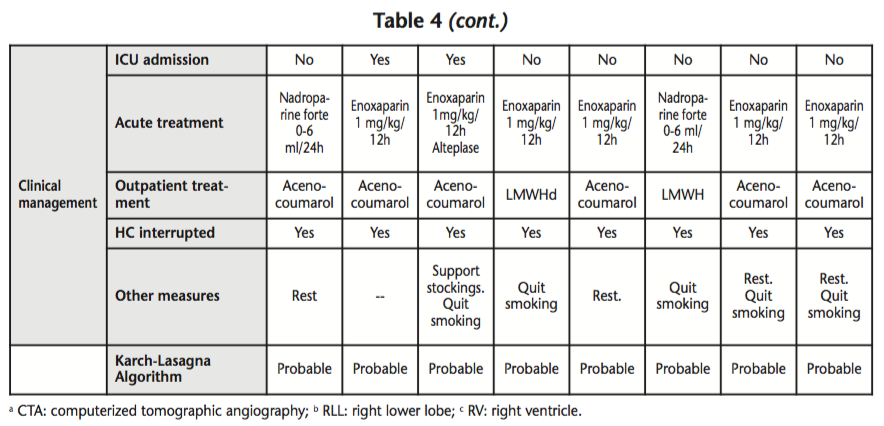
During the period between July, 2012 and July, 2014, eight suspected PEs were detected in the Hospital Pharmacy Unit among patients using HCs who were admitted to hospital. The mean age of patients was 22.9 years (SD: 2.67) and the mean number of RFs was 2.3 per patient (SD: 0.9); seven patients had >2 associated RFs, five patients were smokers, and five patients were obese. The HC involved was: oral ethinylestradiol/cyproterone (derived of progesterone) in three patients, etonogestrel/ethinylestradiol vaginal ring (3rd generation) in two patients; oral ethinylestradiol/drosperidone (4th generation) in two patients, and oral ethinylestradiol/chlormadinone (derived of progesterone) in one patient. Those data collected appear on table 4. Currently, all eight patients have recovered from their thromboembolic event.
According to data provided by the PC, in the same period of two years, 46 suspected PEs associated to the use of HC were registered in Spain, including those reported from our hospital (17%).
DISCUSSION
Despite the fact that the risk of PE associated to taking HC is very low, this is a severe ADR which requires hospital admission, and can endanger patients’ lives. A series of eight PE cases in young women has been described, which were detected in the Hospital Pharmacy Unit though the pharmaceutical validation of treatments in hospitalized patients, with a probable association with the use of HC according to the Karch-Lasagna algorithm applied.
In none of the eight cases described, the HC involved was second generation; these are associated with a lower risk of PE. All patients also presented concomitant RFs. These data demonstrate the importance of conducting an adequate anamnesis, evaluating all RFs associated to each patient before initiating treatment with HC and during said treatment, because RFs can vary over time. Likewise, it is necessary to select the most adequate HC for each patient, with the aim of reducing the risk of VTE.
According to the indications by the AEMPS after reviewing the safety of HCs use, it is recommended to use second generation HCs in all patients about to initiate contraceptive treatment; the use of third or fourth generation HCs could be considered in search of a better lipid profile and the lower risk of acute myocardial infarction for those patients who had previously presented situations of high thrombotic risk (such as pregnancy or surgery), and have not suffered VTE.
An adequate health education must be conducted for patients, informing about the potential risks of taking HCs and which RFs will favour PEs, with the aim of reducing as much as possible those already present (such as smoking or excess weight), and preventing or detecting the potential RFs which can appear in the future (such as immobilization). Patients should also be informed about the signs and symptoms of VTE for an early identification.
HCs are drugs which have demonstrated having a benefit in terms of preventing non-desired pregnancies superior to the potential risks associated with their use. Conducting coagulation tests in all patients using HCs would not be justified, because the incidence of VTE associated with the use of HCs is still low, but it could become relevant in the case of patients who have had previous episodes of VTE or a family history of VTE.
The limitations of our study are its retrospective nature, where the indication of ethinylestradiol-cyproterone could not be determined in one of the patients, the arterial blood gas tests were not available for all patients, and the duration of treatment with HCs until the thrombotic event could not be established, in order to determine whether the PE occurred within the first year of treatment.
Competing interests: The authors declare no conflicts of interest.
BIBLIOGRAPHY
1. Uresandi F, Blanquer J, Conget F, de Gregorio MA, Lobo JL, Otero R, et al. Guidelines for the diagnosis, treatment, and follow-up of pulmonary embolism. Arch Bronconeumol. 2004 Dec;40(12):580-594.
2. Giménez S, Shenguelia L, Yuste E, Carrasco E, Verdú I. Manual de referencia SEMERGEN ETV. Madrid: SEMERGEN & SCM. 2006.
3. Samama MM, Dahl OE, Quinlan DJ, Mismetti P, Rosencher N. Quantification of risk factors for venous thromboembolism: a preliminary study for the development of a risk assessment tool. Haematologica 2003 Dec;88(12):1410-1421.
4. Heit JA. Risk factors for venous thromboembolism. Clin Chest Med. 2003 Mar;24(1):1-12.
5. Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008 Jan 1;117(1):93-102.
6. González-Paredes A, Rodríguez-Oliver A, Fernández-Parra J. Enfermedad tromboembólica y anticoncepción. Actualización Obstetricia y Ginecología 2010. Editorial: J. Fernández Parra, F. Montoya Ventoso. 978-84-693-16.
7. Pérez-Campos EF. En: Introducción. Breve historia, situación y futuro de la anticoncepción hormonal combinada (AHC). [citado 02 agosto 2014]. Disponible en: sec.es/descargas/AH_Anticoncepcion_Hormonal_Combinada.pdf.
8. Anticonceptivos hormonales combinados: conclusiones de la revisión del riesgo de tromboembolismo venoso (información para profesionales sanitarios). AEMPS. Referencia: MUH (FV), 27/2013. [citado 02 agosto 2014]. Disponible en: http://www.aemps.gob. es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2013/NI-MUH_FV_27-2013-anticonceptivos.htm.
9. Estelles-Cortes A, Gilabert-Estelles J. Hormonal oral contraceptives, coagulation and thrombosis. Rev Clin Esp. 2001 Dec;201(12):681-684.
10. Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011 Apr 21;342:d2151.
11. Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011 Apr 21;342:d2139.
12. Spitzer WO. Cyproterone acetate with ethinylestradiol as a risk factor for venous thromboembolism: an epidemiological evaluation. Journal of obstetrics and gynaecology Canada: JOGC= Journal d’obstétrique et gynécologie du Canada: JOGC, 2003, vol. 25, no 12, p. 1011-1018.
13. Sánchez-Borrego, R. La importancia de la elección del gestágeno. En: TSH: Nuevos datos, nuevas estrategias: X Congreso Nacional de la AEEM y Sección de Menopausia de la SEGO. Barcelona: Schering España, S.A; 2008. 39-41.
14. Gilabert J. Anticoncepción hormonal oral y hemostasia. En: Conferencia de Consenso. Prescripción y manejo de anticonceptivos hormonales orales. [citado 10 septiembre 2014]. Disponible en: http://www.schering. es/varios/publicaciones/conferencia consenso/conferenciaconsenso.pdf.
15. Inicio de la revisión de la seguridad de los anticonceptivos orales combinados de tercera y cuarta generación. AEMPS. Referencia: MUH (FV), 06/2013. [citado 02 agosto 2014]. Disponible en: http://www.aemps. gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2013/NI-MUH_FV_06-2013-anticonceptivos.htm.
16. Inicio de la revisión del balance beneficio-riesgo de los medicamentos que contienen acetato de ciproterona en combinación con etinilestradiol. AEMPS. Referencia: MUH (FV), 07/2013. [citado 19 abril 2016]. Disponible en: http://www.aemps.es/informa/notasInformativas/ medicamentosUsoHumano/seguridad/2013/docs/NI-MUH_FV_07-2013-etinilestradiol.pdf.
17. Medicamentos que contienen acetato de ciproterona en combinación con etinilestradiol. Actualización de sus condiciones de autorización. AEMPS. Referencia: MUH (FV), 12/2013. [citado 19 abril 2016]. Disponible en: http://www.aemps.es/informa/notasInformativas/ medicamentosUsoHumano/seguridad/2013/docs/NI-MUH_FV_12-2013-ciproterona.pdf.
18. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981, 30: 239-4.
____
Download PDF: Risk of pulmonary embolism associated to the use of hormonal contraceptives: a series of eight cases
